The physical properties of zeolites make them extremely important in many industrial processes. This one-page reference provides information on the structure and chemistry of zeolites that allows their use in the chemical process industries (CPI).
Zeolite structure
Zeolites are a class of hydrated aluminum silicates made up of silica and alumina tetrahedra (SiO4 and AlO4 linked in a tetrahedral configuration). The linked tetrahedra form complex three-dimensional structures with large, cage-like cavities and channels (Figure 1). Zeolites have open, non-dense structures — 20–50% of a zeolite’s volume is void space. Zeolites’ uniform framework of pores can be exploited to control chemical reactions by adsorbing reactant molecules within the zeolite pores. Certain reaction pathways will be favored based on the shape and chemical composition of the zeolite pores.
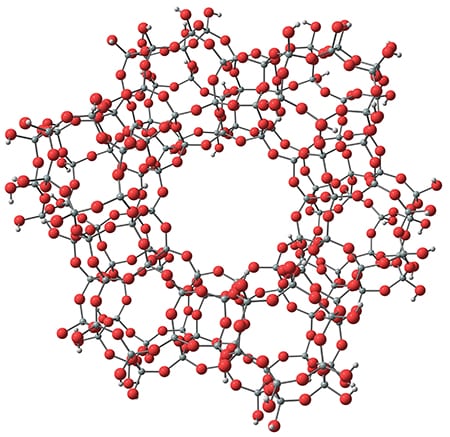
Figure 1. The linked silica and alumina tetrahedra of zeolites create cage-like cavities and channels that can be exploited to control chemical reactions
There are 45 types of naturally occurring zeolites, but their structures and properties are limited from an industrial perspective. Most industrial uses employ one or more of the close to 200 synthetic zeolites that currently exist. The International Zeolite Association (www.iza-online.org) Structure Commission assigns a three-letter code for each class of zeolites, corresponding to its three-dimensional crystal structure.
Synthetic zeolites allow control of a wider range of properties, including Si-to-Al ratio, pore size and geometry, and the ability to incorporate metals and other elements into the active sites. Most synthetic zeolites are made using a process of slow crystallization of a silica-alumina gel in the presence of alkalis and organic templates. Many possible zeolite structures are theorized.
Major industrial uses
Zeolites are widely used in the CPI; the majority in the following three general areas:
Catalysis. Because zeolites are cation exchangers, it is possible to introduce a variety of cations with different catalytic properties into the zeolite’s crystal structure. Simultaneously, their rigid pore geometry serves as a steric influence on reactions, where the pore size and shape controls the access of reactants and products. By controlling the pore size and architecture, engineers can suppress formation of undesired products, for example. Zeolites serve as catalysts for many important reactions involving organic molecules in the petroleum refining and petrochemical sectors (see Table 1).
Adsorption and separation. Zeolites’ porous crystalline structure can be exploited as a molecular sieve, where molecules with certain properties are preferentially adsorbed. Zeolites can be designed to separate molecules according to differences in size, shape and polarity, making them useful as dessicants and in gas separation.
Ion exchange. Hydrated cations within the zeolite pores are bound loosely to the zeolite framework, and can exchange with other cations in aqueous media. This ability makes zeolites useful as industrial water-softeners, where sodium and potassium ions in the zeolite are exchanged for calcium and magnesium ions in the water. Also, zeolites can be used to remove radioactive or toxic heavy-metal cations from liquid nuclear waste and groundwater, respectively.
Catalysis chemistry
In addition to cations, zeolites can have protons bound into their framework. This molecular structure gives rise to high acidity, which can be useful in many key organic reactions. Protons can act as Brønsted acids (proton donors) in reactions with hydrocarbons that form carbocations, for example.
Selected references
1. Baerlocher, C., McCuskar, L.B., and others, “Atlas of Zeolite Framework Types,” 6th ed., Elsevier, Amsterdam, The Netherlands, 2007.
2. Martinez, C. and Perez-Pariente, J. (editors), “Zeolites and Ordered Porous Solids: Fundamentals and Applications,” Valencia (Spain) Polytechnic Institute, 2011.
3. Peskov, M., Zeolites, University of Stockholm, www.asdn.net, accessed May 2017.