The industrial uses of onsite-generated ozone are continuously growing. This article outlines applications for ozone in the CPI and discusses the advantages and disadvantages of using this gaseous chemical
Ozone is most commonly thought of as the stratospheric barrier to ultraviolet radiation on its way from the sun to the earth’s surface. It is also commonly thought of as a ground-level pollutant and component of smog. But ozone is also a powerful chemical oxidizer that can be harnessed and used in various industry sectors for a host of applications. This article outlines several of the most important industrial uses of ozone, and evaluates the benefits of using the chemical against the risks associated with its potential hazards. In the past, several factors surrounding the onsite generation of ozone, along with the efficiency of generation processes and the ability to operate and control them remotely, have prevented widespread adoption of ozone use. Now, several leading companies have refined and advanced ozone generation, making industrial use more feasible for an increasing number of applications.
Ozone foundations
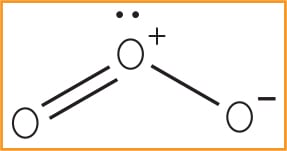
Chemically, the prominent characteristics of ozone are its instability and high oxidation potential, which make ozone a powerful oxidizer. Oxidation involves the loss of an electron and a change of valence, due to the presence of oxygen. Detectable at 10 parts per million (ppm), this pungent, primarily colorless gas (although sometimes thought to be light blue at higher concentrations) is indiscriminate in its reactions, but has a redox potential of 2.07, making it incredibly powerful. As the triatomic, allotropic form of oxygen it has a molecular weight of 48 and is recognized by the chemical symbol O3 (Figure 1). The chemical condenses to a dark blue liquid and crystallizes to a bluish black color.
In nature, ozone is formed by the reaction of oxygen molecules that have been energized by solar radiation in the stratosphere. Ozone is also formed after lightning discharges in the atmosphere. Tropospheric ozone is created by chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOCs). This type of ozone is considered ground-level ozone, and is caused by industrial pollutants and other contaminants reacting with sunlight. In this case, ozone is a component of smog, and can cause public health problems.
In industrial applications, ozone, sometimes called “activated oxygen,” is generated by exciting a flow of oxygen with high amounts of energy or ultraviolet or other optical energy. This will cause decomposition of the oxygen atoms, causing diatomic oxygen to split and recombine with other O2 molecules nearby. Because the source of ozone for use in industrial applications is ambient air, it can be a sustainable chemical treatment for many industrial scenarios In general, ozone formation occurs according to the following equations:
O2 + Energy ——> O· + O· (oxygen free radicals) (1)
O2 + O· ——> O3 (2)
Industrial ozone
The list of industries using ozone grows daily. Over 100 industries actively use ozone in thousands of applications. Many of these involve water treatment, including aquaculture, aquariums, waterparks and others. Ozone is also used in manufacturing processes for a range of products, such as deodorant, and in the equipment used by dentists.
Many pools, spas and hot tub manufacturers have added ozone as a secondary disinfection method, ensuring that customers equipment runs efficiently, and less chemical is required. Zoos and aquariums have added ozone systems to water sources to keep water sources disinfected and boost the dissolved oxygen in the water. Ozone also helps remove the TDS (total dissolved solids) and TSS (total suspended solids) by helping coagulation of the particulate. Harmful ammonia and nitrates are oxidized to the less harmful nitrates keeping animals healthier.
Ozone compound is categorized as a disinfection agent, a sporicidal agent, an algaecide and a viricide. Disinfection is the process of cleaning something to remove microorganisms such as bacteria, protozoa, and cysts. Due to its extreme instability and high oxidation potential, ozone is very powerful and indiscriminate in terms of reactivity with other chemical species. Ozone has been shown as an effective treatment for destruction of VOCs, removal of metals, total suspended solids, organic carbon and many, many more as well as significant reductions to chemical oxidation demand (COD).
Its high instability under standard pressure and temperature requires onsite generation for industrial applications (Figure 2). Its high reactivity and short half-life means that long-term storage is not an option, and industrial systems must be designed for on-demand treatment.
Under typical treatment conditions, using a relatively pure oxygen source stream and a corona discharge chamber design, which uses a high-voltage electrical arc, the reactions shown in Equations (1) and (2) can produce up to 9–12 wt.% ozone, although typically output is in 1–9 wt.% ozone. The remainder of the stream is left as oxygen. The concentration of ozone is limited to this range.
As ozone concentrations rise above this concentration, the destruction reaction becomes more frequent, thus returning greater quantities to O2 and maintaining this equilibrium. This instability is also the reason ozone cannot be stored and must be generated immediately prior to application. Ozone must be dissolved in water. The three most popular methods of introducing ozone into water are direct injection, venturi injection or bubble diffusion. Once dissolved in the water, the free-radical oxygen atom is released to oxidize other molecules.
Ozone applications
Disinfection
One of the primary uses of ozone in water and wastewater is disinfection. Plants are moving from traditional oxidation to ozone on the front end and adding chlorination on the end for residual only. When ozone breaks down in water, it forms the free radicals hydroxyl (·OH) and hydroperoxy (HO2). It is the reaction with these radicals, along with protoplasmic oxidation that play a major part in the effective disinfection process. Damage to the RNA of the bacteria (purines and pyrimidines) occurs with the cell lysis.
As with any disinfection process, effectiveness is dependent on many factors. Contact time, type of organism and ozone dosage all factor into the success criteria and must be considered when looking at treatment options. Typically ozone can achieve a 2–4-log reduction in under one minute.
One of the largest increases in the use of ozone has come in the hydraulic fracturing (fracking) industry, where ozone has been used to disinfect water for reuse and eliminate the need for additional chemical treatments ozone offsets the need for biocides, and anti-scalants. With onsite generation (typically in mobile trailers) producers reduce transportation cost, safety concerns from chemical spills, while having movable treatment on demand.
Ozone does not create disinfection byproducts when it reacts. In freshwater the half-life of ozone is typically ten to twenty minutes, whereas in wastewater, ozone has been documented as being entirely consumed within 8.6 seconds. This is due to the extreme amount of potential reactants that are present in wastewater. It is also highly effective at the removal of pharmaceuticals in water.
Color removal/abatement
Water appears to have color when light is reflected off suspended solids or when a dissolved material causes a break in light or adsorption of radiation. While color causing materials can be filtered out colors that are caused by aromatic structures (poly, or substituted), perplexed ions, and other molecules that utilize conjugate bonds take a bit more to remove. Typically, these come from industrial applications and require an oxidative reaction to remove the color from the stream.
Organics play another role in taste and color in groundwater. The primary source of taste and color in groundwater and surface water comes from tannins.
Color removal effectiveness in water depends not only on the chemistry and dosage but on the temperature as well. The best results for color removal are achieved by treating the water/wastewater for biological oxygen demand (BOD) and chemical oxygen demand (COD) prior to ozone treatment, so there is little interference with the “consumption” of ozone. Treatment occurs best at low temperatures.
Odor control
The primary source of odor in wastewater comes from the production of hydrogen sulfide (H2S) by sulfate-reducing bacteria (SRBs). Sulfate reducing bacteria produce hydrogen sulfide as part of their metabolic process in an anaerobic environment. As well as causing an unpleasant odor, concentrated sulfides can be dangers, even in low concentration. Traditionally, plants turn to chemicals such as calcium nitrate to control the odor. This does not kill the bacteria but changes their metabolic process to utilize the nitrate instead of sulfides. Over time, the bacteria adapt to the chemical and the effectiveness is lost until the dosage is adjusted. The number of bacterial colonies also grows adding to the chemical demand.
Ozone provides a different approach to treating the odor. As anaerobic bacteria, the SRBs cannot survive in an aerobic environment. By destroying the bacteria and changing the habitat to an aerobic environment (ozone reduces to oxygen) the bacterial colonies can no longer survive not only reducing the odor but preventing new bacteria from colonizing. Most ozone treatment is applied at the lift station in the vapor space. Oxygen/ozone is able to breach the biofilm in hard to reach places that chemical cannot penetrate. Typically, an additional oxygen source is added to the force main to continue the process. Ozone applied in a force main acts as a jumpstart to additional treatment but has little effect past the injection point due to the organic nature of wastewater and the amount to BOD and COD in the water.
The concept of using ozone in odor control is also effective in wastewater tanks and in petroleum refinery offgassing. Ozone is used in the destruction of the volatile organics such as benzene, formaldehyde and hydrogen sulfide.
CIP in food and beverage
There are many benefits for the use of ozone in food and beverage. On-demand treatment allows plants the flexibility in work flow without having to worry about chemical shortages or equipment maintenance. Ozone’s ability to eliminate bacterial on contact, as well as eliminate biofilm offers piece of mind to producers. Since its approval for food production in 2001, it has gained popularity in industries such as dairy, beverage and produce, where the elimination of bacteria without an effect on taste or smell is of the utmost importance. The primary focus of ozone in the food and beverage industry has been in the clean in place (CIP) systems and in the wastewater treatment.
Ozone in CIP systems can replace the need for hot water cycles. It can be added to the rinse cycle, eliminating the need for the “sanitation” cycle, shortening the CIP cycle time, reducing water and energy costs and eliminating the need for the “sanitation” chemicals. In addition, there is no residual left behind, so product contamination due to cleaning or rinsing chemicals is eliminated.
In dairy, ozone is finding popularity not only in the processing plants but on the farms, where small generators can provide CIP disinfection to the pipelines between individual milking stations and the bulk tanks. Traditionally this cleaning process takes place two to three times per day and involved hot water and chemicals. Ozone has reduced the need for hot water and harsh chemicals while reducing cost.
Surface sanitation
Ozone has proved effective for surface sanitation in food plants and other industries where a secondary process is necessary to ensure cleanliness. Over time, bacteria can develop a resistance to chlorine. Regulations on residuals and can make dosing to overcome this resistance difficult for discharge or recirculation. Materials of construction can also be a factor. Chlorine can also leave an odor and impact taste. Ozone eliminates all this issue by leaving only an oxygen residual with no taste or smell.
Cooling tower disinfection
Cooling towers in large plants can be the number one user of water. Typical cooling-tower operation allows a bleed of water into a wastewater stream. This water contains chemicals such as corrosion inhibitors and anti-scalants used to optimize the operation of the tower. Studies and field applications have shown that using ozone in cooling towers is more effective than traditional chemicals, increasing the mass transfer of the chiller by over 20%, with no chemical residual to monitor. The oxidizer disinfects the cooling tower, eliminates biofilm build up, decomposes organic waste, removes carbonate scaling and reduces corrosion caused by bacterial sources even in copper heat exchangers. As ozone reduces to oxygen, there is no danger in off gassing, or issue in using it in open air cooling towers.
Wet scrubber
Removing pollutants and odors from process exhaust is an important and challenging part of industry. Wet scrubbers have become instrumental in industries producing volatile organics in the exhaust of their processed. Wet scrubbers can be effective but become more efficient with ozone. By injecting ozone into the sump of the scrubber, Ozone in the water bonds to the carbon in the complex chain and converts it into CO2 in the tower. There can also be a synergetic effect of the ozone and oxygen in the scrubber environment, keeping additional contaminants oxidized and scrubber wall free from biofilm and other contaminants.
Ozonated water in fire restoration and mold remediation
Ozonated water destroys odor and smoke on contact. Infused water sprayed on carpet, furniture and in other hard to disinfect areas, such as ventilation ducts, are deodorized in seconds. The residual oxygen brightens the air and leaves no dangerous fumes.
In mold remediation, the oxidation/disinfection power of ozone is able to kill mold in hard to reach places. It is common practice to use ozone as a disinfection both as a mist and a fog to kill mold after water emergencies caused by flooding and hurricanes.
Iron and manganese oxidation
Without oxidation, Fe(II) or ferrous iron and Mn(II) also known as soluble manganese will pass through mechanical filtration, making it hard to remove from water sources. Both must be oxidized before filtration can occur. Conventional oxidizers, such as permanganate and chlorine/sodium hypochlorite, have been the traditionally chosen method of oxidation, but these methods offer increasing chemical costs, residuals and safety issues to monitor, and long contact times.
The reaction time between ozone and Fe(II) is incredibly quick at low dosing, offering hydrolyzed iron at around 0.43 mg/mg of Fe(II). This reaction will occur before the manganese reaction if both are present in the water. Mn(II) and ozone react fully at about 0.88 mg/mg of Mn(II). This reaction takes longer, and must be watched carefully. Over-oxidized Mn(II) can become Manganese permanganate (MnO4). This reaction is not a longterm one, and will usually reverse itself in the longterm.
Ozone in comparison
In comparison to other methods of disinfection, ozone offers several benefits. Ozone can replace other chemicals. Its flexibility offers advantages far beyond cost. It works in areas where other disinfection cannot.
Chlorine and chloramines requires contact time. Bacteria, viruses and cysts must be replicating or feeding for treatment to be effective. This can take up to 140 minutes. Typical drinking water contact time for a two- to four-log reduction takes three to six minutes per liter. For Ozone this contact time is 30 seconds to a minute.
Chlorine and chloramines cannot, however, be used to deactivate protozoa. According to the EPA, the Ct-value for deactivation by chlorine averages between 3,000 and 4,000 mg min/L for 90% deactivation. With adequate dosage and contact time, Ozone is an effective disinfection against protozoa, although the contact time is still much longer than that of bacteria or viruses.
Ozone is very powerful, in some cases, may not be used alone for disinfection. When a measurable residual must be maintained, a secondary source of disinfection must be added to a process. In these cases, chlorine, or chloramine may be added, if there is little danger of trihalomethane formation.
Although ozone is an oxidizer, many applications see their risk of corrosion reduced in comparison to other chemicals. There is two reasons for this. The first is in many cases, a bacterial source of the corrosion. The second is a need to overdose harmful chemicals to overcome the bacteria in a system.
As bacteria colonize, they form a “slime” layer called biofilm. This film is an extracellular matrix in which the microorganism are embedded in an extracellular matrix to protects the colonies from harm. Within this environment, the metabolic processes of the bacteria can be detrimental to the host structure materials of construction. In many cases, this damage goes undetected as the biofilm “fills the holes”. Once this layer is removed, the damage is apparent. Most liquid disinfection processes cannot remove biofilm, only impede the growth. An additional chemical is needed to remove the film. Gaseous disinfection such as ozone, oxygen and chlorine dioxide are able to cut through the biofilm, and reach the bacteria protected behind it.
Advantages of ozone
With its strong oxidizing qualities and quick reaction time, ozone is capable of multiple reactions in one application. Its residual is pure oxygen, raising dissolved oxygen levels in the host liquid. Most reactions take place in seconds. With a solubility twelve times that of oxygen, it reduces water surface tension and aids in many treatment processes. Ozone is lighter than oxygen, making it helpful in flocculation applications. Its ability to break down complex organics and aid bioremediation is incredible. In recent years, it has been added to many traditional technologies, such as dissolved air flotation (DAF) and biofiltration, to better performance. Ozone leaves no residual and creates fewer harmful disinfection byproducts that can be caused from other disinfection methods such as chlorination. Contact time can be varied.
Disadvantages of ozone
In the past, the biggest drawbacks to ozone disinfection surround costs. Ozone must be generated onsite. The initial investment to install the ozone systems can be much more capital intensive than other chemical treatment processes. Although high voltage is no longer required to produce ozone, an energy source is required. An ozone generator will require more electricity to run than a standard chemical pump. Currently, several companies have developed intellectual property that has lead to breakthroughs on cost and efficiency, now enabling economical use with strong internal rate of return compared to traditional chemical approaches.
Materials of construction must be addressed when looking at installing an ozone system. As with any strong oxidizer, not all metals and plastics are compatible.
Ozone’s residual effects are limited. Injected into a system with high organics, its ability to stay stable is very short, therefore it cannot provide a long-term residual the way chlorine or chlorine dioxide can. This can be very beneficial in cases where a longterm residual can be damaging or is unwarranted.
Ozone systems must consider all constituents of the host water to ensure proper reactions. The strong oxidative qualities can produce unwanted effects, especially in industrial wastewater. It is recommended that a complete water chemistry analysis is done at least once a year to prevent unwanted reactions.
Working with a world-class ozone provider can overcome all the challenge of selecting the correct system thanks to system engineering, generation efficiency, and proprietary dosing systems.
Ozone safety
Ozone, like other oxidizers, must be handled with caution. Proper materials of construction must be used in areas that will come in contact with ozone. Reaction time with ozone is instantaneous. Handled incorrectly, or carelessly, cell damage can happen immediately.
Ozone’s safety is gaining recognition. At low doses, ozone therapy in hospitals is a recognized form of disinfection treatment to assist in the body’s intake and use of oxygen. Ozone was granted GRAS (generally recognized as safe) status in the U.S. Food and Drug Administration (FDA) rule June 15, 2001, for use on food products. Ozone has also been approved and deemed safe by the following organizations: NSF International, U.S. Department of Agriculture, U.S. Centers for Disease Control and Prevention (CDC) and by the National Swimming Pool Association.
Final thoughts
With recent advancements in its generation, ozone can offset or replace many of the commonly used chemicals in water and wastewater. Its powerful oxidation can provide massive benefits when applied correctly. Water chemistry and materials of construction must be considered when reviewing ozone’s applicability. Many lists exist showing the chemical compatibility of ozone and its oxidation power. (Example: https://www.ozonesolutions.com/info/ozone-compatible-materials). The power of ozone is still being explored. New applications for this constituent are being developed and explored every day.
Edited by Scott Jenkins
Author
Tonya Chandler is the vice president of sales and marketing for Anue Water Technologies Inc. (5123 South Royal Atlanta Drive, Tucker, GA 30084; Email: [email protected] ; Phone: 760-213-7739). Anue provides ozone and oxygen solutions to customers for both municipal and industrial applications.
Sources
https://www.epa.gov/sites/production/files/2016-12/documents/mcchristian-ozone.pdf
http://www.nesc.wvu.edu/pdf/dw/publications/ontap/2009_tb/ozone_DWFSOM44.pdf
https://www.wwdmag.com/disinfection/overview-ozone-water-wastewater-treatment
https://pubchem.ncbi.nlm.nih.gov/compound/ozone#section=Top
Utilizing Sustainable Treatment Gases: Oxygen and Ozone. Modern Pumping
https://www.lenntech.com/library/ozone/comparison/ozone-disinfectants-comparison.htm
https://www.ozonesolutions.com
Jim Baker, Primozone
Paul Turgeon, Anue Water Technologies inc
Avantika, Anue Water Technologies Inc.