This column is based on “Sodium Chlorate Production – Cost Analysis,” a report published by Intratec. It can be found at: www.intratec.us/analysis/sodium-chlorate-production-cost.
Sodium chlorate (NaClO3; also known as chlorate of soda) is an odorless inorganic salt with colorless crystals and a cooling/saline taste. The compound is soluble in water and most organic solvents. The main uses of sodium chlorate derive from its oxidizing characteristics, and its main application is as a bleaching agent in a variety of processes. Because of its high oxidizing power, sodium chlorate is generally not found naturally. Its first uses date to the beginning of the 20th century, however, its industrial production only began at the end of the 1960s.
The commercial-scale synthesis of sodium chlorate is mainly based on the electrolysis of a hot sodium chloride solution — similar to the widely used chlor-alkali processes. The main difference lies in the fact that there is no need to prevent the reaction between chlorine and hydroxide ions. The on-purpose synthesis of sodium chlorate through chemical reactions is not commonly employed on industrial scale.
The process
The present analysis discusses an industrial process for sodium chlorate production. The process comprises two major sections: (1) electrolysis and (2) sodium chlorate recovery (Figure 1).
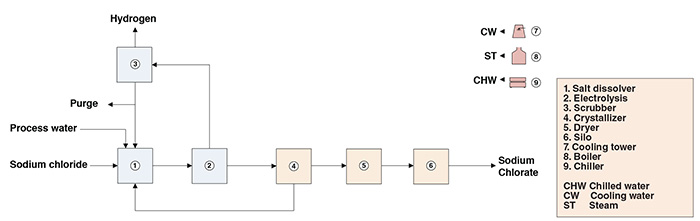
Figure 1. The diagram shows the production process for sodium chlorate
Electrolysis. In a salt dissolver, a brine solution is prepared from sodium chloride raw material and process water. After purification of the NaCl solution, hydrochloric acid and sodium dichromate are added (to increase cell efficiency and to protect electrodes from corrosion, respectively). The purified, acidified solution is then electrolyzed. At the cathodes, hydrogen gas is generated and released along with impregnated chlorine. The released gas is collected in a column that prevents chlorine emissions through caustic scrubbing. At the anodes, chlorine atoms generated from the electrolysis combine with water and free ions to form hypochlorous acid and sodium hypochlorite. Hypochlorous acid and sodium hypochlorite then react to form sodium chlorate. Reaction tanks downstream from the electrolytic cells provide a longer residence time for the chemical reaction between hypochlorous acid and sodium hypochlorite to take place. The liquor from the electrochemical cell is finally transferred to the crystallizer in the sodium chlorate recovery section.
Sodium chlorate recovery. Prior to crystallization, residual hypochlorites are eliminated through heating and addition of chemicals. The cells’ liquor, virtually free of hypochlorites, is heated, subjected to vacuum evaporation and finally crystallized under vacuum. The sodium chlorate crystals are recovered by centrifugation, washed and fed to a dryer. Dry sodium chlorate is conveyed to silos prior to being packed into bulk bags.
Production pathways
Industrial-scale production of sodium chlorate is primarily based on brine electrolysis. Figure 2 presents different pathways for the production of sodium chlorate.
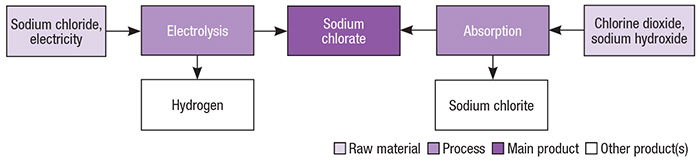
Figure 2. Brine electrolysis is the primary production pathway for sodium chlorate, but others exist
Economic performance
The total operating cost (raw materials, utilities, fixed costs and depreciation costs) estimated to produce sodium chlorate was about $440 per ton of sodium chlorate in the fourth quarter of 2016. The analysis was based on a plant constructed in the U.S. with the capacity to produce 80,000 metric tons per year of sodium chlorate.
Edited by Scott Jenkins
Editor’s note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.