Nitrogen (N2) makes up 78% of ambient air (20.95% of air is oxygen). N2 is an inert (non-flammable) gas used in industrial plants to prevent undesirable chemical reactions from occurring. Nitrogen is the most common inert gas used in the chemical process industries, due to its high natural abundance and low relative cost.
Major uses of N2 include several in metallurgy: for heat treatments; as an alloy constituent; for scavenging; and as a protective gas; as well as in heat treatment. N2 is also used as an inert gas in shielding and firefighting, in food storage and in processing. Nitrogen can be used in the manufacture of other products, including ammonia.
Most N2 is produced onsite at industrial facilities, for local consumption. However, it may be stored and transported as a gas or a liquid, at cryogenic temperatures. High-pressure cylinders or cylinder clusters can be used for storage and shipping of small quantities of nitrogen gas. Liquid nitrogen can be transported and shipped in specially insulated storage vessels (small quantities) or by road and rail tankers (large quantities).
Production process
The process of recovering N2 from air comprises three major sections: (1) purification; (2) refrigeration; and (3) rectification (Figure 1).
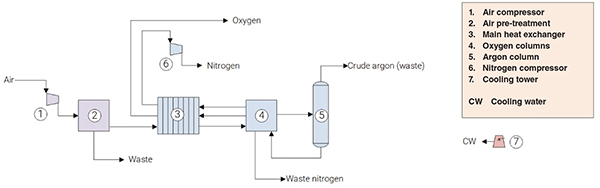
FIGURE 1. The diagram shows the process for producing nitrogen from ambient air
Purification. Initially, the air is purified from dust particles, carbon dioxide and water. This is accomplished by compressing the air, passing it through a heat exchanger (causing water condensation) and then through an adsorbent bed of molecular sieve for the removal of water, carbon dioxide and traces of other impurities.
Refrigeration. At this point, the purified air is cooled down/liquefied, as required for rectification steps downstream. This step is conducted in a plate-fin heat exchanger, using refrigeration values contained in the products (oxygen, nitrogen) and waste (nitrogen) streams.
Rectification. The last step in the process consists in submitting the cooled/liquified air to a fractional distillation carried out in two columns for nitrogen-oxygen separation, operating at different pressures, in such a way that nitrogen is distilled as a vapor and oxygen is obtained as a liquid. In addition, while oxygen and argon have close boiling points, a third column is used exclusively for the removal of that contaminant, vented to the atmosphere.
The main product obtained in the process under analysis here is gaseous, high-purity N2 (the product gas is greater than 99.7% N2), at 60 bars absolute.
Production pathways
Millions of tons of N2 are isolated from atmospheric air every year, through different separation processes that are selected according to the production scale and purity required (Figure 2). The production of large quantities of relatively pure nitrogen is carried out, mostly, through cryogenic distillation. On the other hand, moderate volumes of low-purity nitrogen are commonly produced via pressure-swing adsorption and membrane permeation.
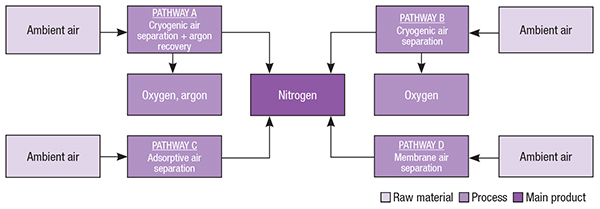
FIGURE 2. Cryogenic separation is used to produce gases from air, but other routes exist
In addition to nitrogen, oxygen (O2) and argon (Ar) can also be generated commercially in air-separation processes using cryogenic distillation and argon recovery units. Oxygen is also produced by vacuum-swing adsorption (VSA).
Edited by Scott Jenkins
Editor’s note: Content for this column was originally developed by Intratec Solutions LLC (Houston; www.intratec.us) and is edited by Chemical Engineering. The analyses presented are based on publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing the analyses can be found, along with terms of use, at www.intratec.us/che.