This column is based on “Butyl Acrylate Production from Acrylic Acid and Butanol – Cost Analysis,” a report published by Intratec. It can be found at: www.intratec.us/analysis/butyl-acrylate-production-cost.
Butyl acrylate, the butyl ester of acrylic acid, is among the most industrially important acrylates (along with methyl acrylate and ethyl acrylate). The major use of butyl acrylate is in the production of acrylic polymers, and for making copolymers with polyethylene. It is also used in the formulation of paints, sealants, cleaning products and adhesives, as well as in amphoteric surfactants, aqueous resins, antioxidant agents, elastomers and dispersions for textiles and papers.
Butyl acrylate can be produced from several reactions involving acetylene, 1-butyl alcohol, carbon monoxide, nickel carbonyl, and hydrochloric acid among other chemicals. On an industrial scale, butyl acrylate is produced from ester-grade acrylic acid and butanol, typically in plants that are integrated with acrylic acid facilities.
The process
The present analysis discusses an industrial process for butyl acrylate production. The process comprises two major sections: (1) esterification; and (2) purification (Figure 1).
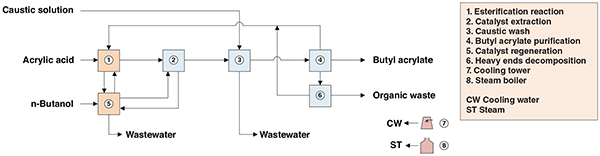
Figure 1. The diagram represents a process for the production of butyl acrylate via conventional esterification
Esterification. Acrylic acid, a small excess of butanol and p-toluene sulfonic acid catalyst are fed to the reaction system. The esterification reactor is connected to a distillation system for continuous removal of water from the reactor medium. This improves the reaction kinetics and shifts the reaction toward ester formation. Organic compounds recovered in the bottoms are recycled to the esterification reactor, while water is used as a solvent for catalyst extraction.
Purification. Recovered water is fed to a catalyst extraction column to separate catalyst from previously cooled reaction product withdrawn from the second reactor. The catalyst stream is recycled to the esterification reactor. The crude product is fed to a wash column, where residues of acrylic acid and catalyst are neutralized with a caustic solution and separated from the crude product as the column bottom stream. The top stream is distilled to recover butanol, which is sent to the dehydration distillation column upstream. In the last purification step, a column separates residual organic heavy wastes from the crude butyl acrylate stream, yielding high-purity butyl acrylate as column overhead. The organic heavy material is directed to the decomposer reactor, where extra butyl acrylate is recovered by the catalytic reaction of heavy byproducts.
Production pathways
Butyl acrylate is primarily made from acrylic acid and butanol, in a variety of manufacturing routes that differ according to the sources of raw materials. In this context, typical butyl acrylate production routes are based on acrylic acid manufacturing, mostly via propylene oxidation, and, to a lesser extent, oxidative carbonylation of ethylene (Figure 2).
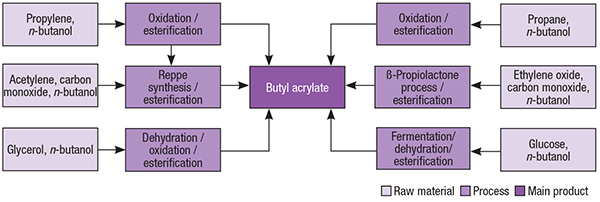
Figure 2. Several different production pathways exist for butyl acrylate
Economic performance
The total operating cost (raw materials, utilities, fixed costs and depreciation costs) estimated to produce butyl acrylate was about $1,400 per ton of butyl acrylate in the first quarter of 2017. The analysis was based on a plant constructed in the U.S. with capacity to produce 150,000 metric ton per year of butyl acrylate.
Edited by Scott Jenkins
Editor’s note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.