The purification of seawater and wastewater for agriculture and human consumption is becoming increasingly important in water-scarce regions. Along with the removal of salt, rendering ocean water and wastewater suitable for humans and plants also involves removal of elements that can be toxic, including mercury, arsenic, lead, uranium, boron and others. While this would usually require several processing steps, a research team led by University of California at Berkeley (www.berkeley.edu) scientists has fabricated a flexible ion-exchange polymer membrane that can selectively adsorb heavy metals at the same time that it removes salt, in a process they call “ion-capture electrodialysis.”
The new technique is designed to be easily added to current membrane-based electrodialysis desalination processes (where electric voltage drives salt ions through the membrane), as well as diffusion dialysis systems. The Berkeley team incorporated porous aromatic framework (PAF) nanoparticles into sulfonated polymer membranes to capture metals contaminating the water.
PAFs are three-dimensional networks of carbon atoms connected by aromatic linkers. Various molecules can be attached to the aromatic linkers to capture specific chemicals. Salts are removed from the water with a series of cation- and anion-exchange membranes, and the embedded PAF particles are selected to capture specific target ions, removing either a single type of valuable ion (gold or copper, for example), or multiple contaminants at the same time, the research team explains (diagram).
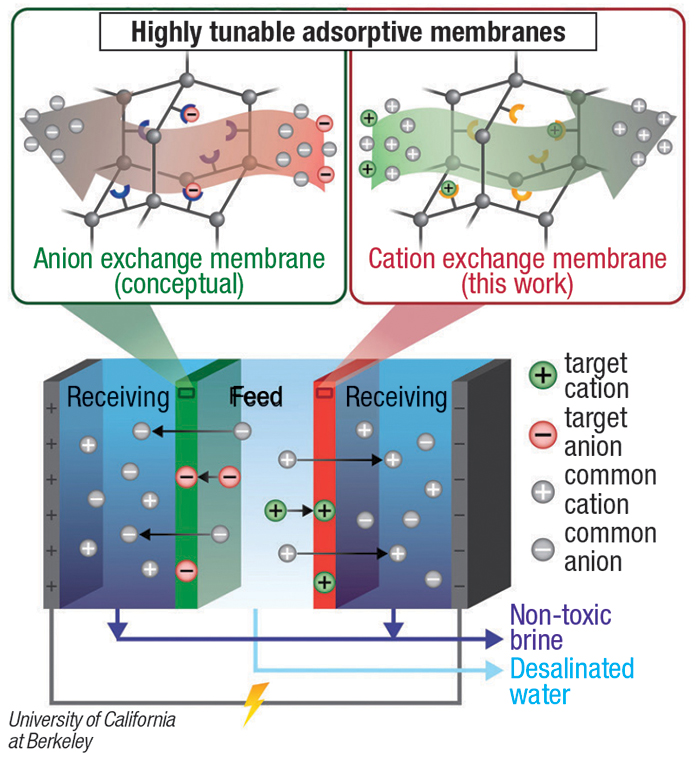
The Berkeley team says the reusable polymer membrane containing the PAF nanoparticles is stable in water and at elevated temperatures, unlike most metal organic frameworks (MOFs), for example. The researchers hope to be able to tune the nanoparticles to remove other types of toxic chemicals, including PFAS (polyfluoroalkyl substances). The group’s work was recently published in the journal Science.