Electrochem Technologies & Materials Inc. (Montreal, Canada; www.electrochem-technologies.com) produced pure electrolytic iron (99.995% Fe) using its patented FerWIN process (diagram) — a sustainable zero-carbon iron-making technology — from ferrous sulfate heptahydrate (copperas) originating from the sulfation of bauxite residues. This pilot test work involved reacting concentrated sulfuric acid with bauxite residues, from which iron, aluminum and sodium sulfates, along with gypsum, are recovered. Then, the electrowinning of iron metal was performed on the crystallized copperas. Pure electrolytic iron flakes were electrowon inside a rectangular electrolyzer with 10 ft2 of cathodes, while regenerating the concentrated sulfuric acid to be recycled upstream during sulfation.
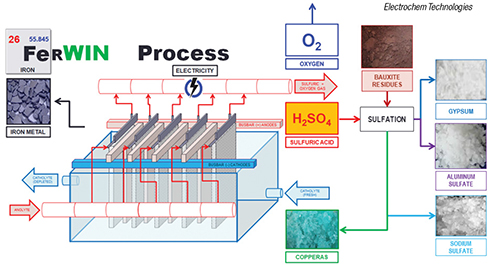
“Based on the excellent faradic current efficiency (98%), low specific-energy consumption (2.9 kWh/kg Fe) and operating expenditures ($315/m.t. of Fe), we are optimistic that combining the sulfation of bauxite residues and the electrowinning of iron could represent a possible route for neutralizing, dewatering, recycling and valorizing red mud and bauxite residues,” says Francois Cardarelli, president of Electrochem Technologies & Materials. “This is particularly true in locations having an oversupply of sulfuric acid from nearby smelters and affordable nuclear power or hydroelectricity,” he says.
From an environmental standpoint, the FerWIN process also releases pure oxygen gas to the atmosphere generating carbon tax credits. The patented technology is now granted and enforced in 16 key jurisdictions (within Canada, China, Japan, South Africa, Europe, Brazil and India) where red-mud landfills represent a serious environmental hazard. As the technology is now technically proven, de-risked, and the costs and benefits analysis favorable, the company is currently seeking to secure licensing agreements for the FerWIN process across the aluminum industry, Cardarelli says.