Decomposing limestone (CaCO3) into quicklime (burnt limestone; CaO) releases significant CO2 emissions, both from the combustion of fuel needed to heat the kiln to temperatures over 1,000°C, and by the release of CO2 from the reaction itself (CaCO3 → CaO + CO2). Because quicklime is a key ingredient in the production of cement, as well as being used in steel, pulp-and-paper and other industries, efforts are underway to reduce the carbon footprint of this operation.
One such effort is the 30-month Decarbonate project that is scheduled to be completed in March. Led by the Technical Research Center of Finland (VTT; Espoo, Finland; www.vttresearch.com) with nearly a dozen industrial partners (see diagram), the Decarbonate project involves the construction of a 12-m, 300-kWe electrically heated rotary kiln to perform precalcination of raw powders for cement, and the production of both quicklime and “lime mud” that is used in pulp mills. Because the kiln is gas-tight, the CO2 released from limestone is nearly pure, so it can either be stored or utilized to make other products. For example, one part of the project will study the conversion of CO2 and H2 into low-carbon gases and Fischer-Tropsch (F-T) liquids. For this step, the H2 is supplied from a 21 kW PEM electrolyzer. The O2 co-product from the electrolyzer supplies an oxygen-enhanced circulating fluidized-bed (oxyCFB) calcinator.
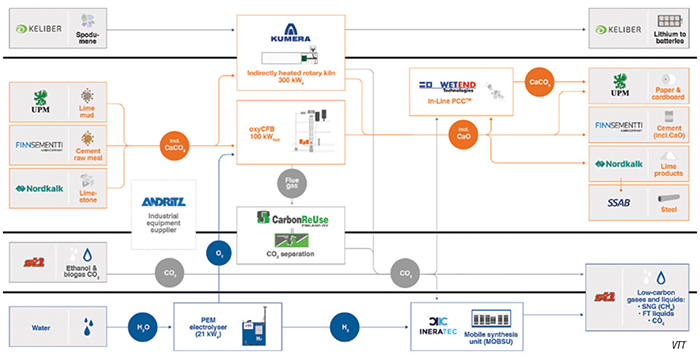
By using low-emission electricity instead of combustion for decomposing CaCO3 — a central part of cement production — and by capturing the CO2 produced in the production process, it is possible to run a cement plant with close to zero CO2 emissions, says VTT.
In the E.U., emissions trading is steering the industry towards reducing emissions. At the current price level, a decrease of 1 metric ton in CO2 emissions means a savings of €60 for the company. For a medium-sized cement plant, for example, a one-third reduction in emissions would mean savings of several million euros per year, says VTT.