Designing a proton-exchange membrane that will balance the efficiency, durability and safety requirements for hydrogen production requires careful consideration of materials and chemistry
Among the ongoing efforts to curb climate change, researchers are actively pursuing the further development of water electrolysis technologies for the production of cleaner hydrogen. “Green” hydrogen can be manufactured through water electrolysis using renewable energy. One method of water electrolysis utilizes a proton-exchange membrane (PEM). Compared to other water electrolysis methods, PEM electrolysis demonstrates several key advantages, including compact design, high electrolytic efficiency, stability and a robust response to fluctuating power sources [1–3]. The mechanics of a PEM electrolysis cell are depicted in Figure 1. Within the electrolysis cell, a PEM, usually less than 200 mm in thickness, separates the anode and cathode. Upon applying voltage, water electrolysis proceeds in the following sequence:
- Water is oxidized at the anode, generating protons and oxygen
- The protons are transported to the counter-electrode (cathode) through a PEM
- Protons are reduced at the cathode, and hydrogen is produced
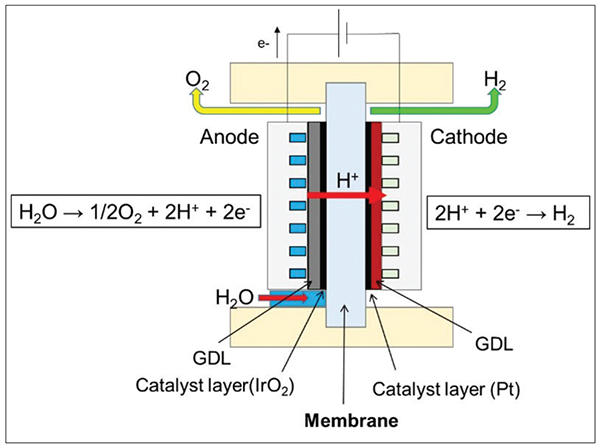
FIGURE 1. A typical structure of a PEM cell for water electrolysis is depicted here [1]
A gas-diffusion layer (GDL) helps to facilitate the efficient release of generated gases during water electrolysis, thus inhibiting bubble adhesion on the catalyst layer. As a result, water can access the catalyst more efficiently at the anode, promoting the oxidation reaction of water.
In industrial applications, hydrogen is utilized as a high-pressure gas. Therefore, two types of compression methods are typically considered for water electrolysis. One involves compressing ambient-pressure hydrogen produced by water electrolysis using a compressor. The other method involves elevating the pressure (to greater than 3 MPa) of the produced hydrogen within a sealed electrolytic cell and cylinder. In the latter method, especially in cases where only the cathode side is pressurized (denoted as differential high-pressure water electrolysis), there is no need for a compressor, which can lead to more efficient hydrogen production [4].
Hydrogen obtained from water electrolysis contains moisture that often must be removed prior to the hydrogen’s end-use application. However, pressurizing hydrogen within water electrolyzers decreases the water content of the generated hydrogen. Therefore, differential high-pressure PEM water electrolysis is a highly efficient and high-purity hydrogen production method.
Making an effective PEM
Reducing production costs for clean hydrogen is a particular challenge. Described in the following sections and in Table 1 are four considerations that can help to reduce costs in electrolysis processes.
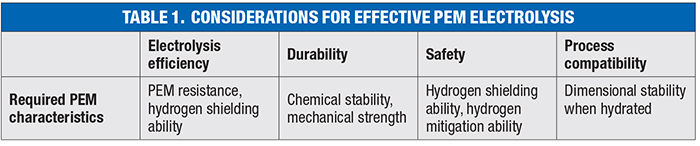
Efficiency. The first consideration is the improvement of electrolysis efficiency, particularly the reduction of cell voltage. Resistance comes from various phenomena, such as oxidation and reduction reactions, proton transport across the PEM interface and mass transport, which dictates water’s access to a catalyst layer. The cell voltage is the total of the voltages derived from these resistances. As the PEM is involved in the proton transport, it is a contributor to total cell voltage, and must be considered for the reduction of resistance that is needed to improve electrolysis efficiency.
Durability. The second design consideration to focus on is ensuring durability that allows for operation over ten years or more. During water electrolysis, hydroxyl radicals are generated through the Fenton reaction of hydrogen peroxide (a side reaction). Because hydroxyl radicals have tremendous oxidative reactivity, PEMs need to have high chemical stability to give them high durability. Also, when performing differential-pressure water electrolysis, the PEM continuously experiences the pressure of the cathode, demanding high mechanical strength.
Safety. The third crucial characteristic to think about is safety. In high-pressure water electrolysis, hydrogen permeates the PEM and mixes with oxygen. The lower explosive limit (LEL) of hydrogen is 4 vol. %. Therefore, it is necessary to enhance the hydrogen-shielding ability and the mitigation ability of the permeated hydrogen. Hydrogen shielding refers to the technically usable amount of hydrogen per the theoretical amount of generated hydrogen. The mitigation ability is the mitigated amount of permeated hydrogen per the amount of permeated hydrogen without a mitigation strategy, such as a gas recombination catalyst (GRC). More details on GRC technologies are described later in this article.
Compatibility. The fourth characteristic is process compatibility. Normally, dry PEMs swell three-dimensionally when hydrated. In-plane expansion significantly affects the yield and quality of PEM manufacturing and subsequent processes. Therefore, high dimensional stability in the in-plane direction is required.
To realize these four characteristics, trade-offs must be considered. For example, reducing PEM thickness is an effective method to reduce membrane resistance, but it is impossible to avoid a decrease in safety due to an increase in the amount of permeated hydrogen. Therefore, developing membrane-design strategies that reduce the need for such trade-offs is a high priority.
PFSA polymers
A perfluorinated sulfonic-acid (PFSA) polymer is the most commonly used polymer for PEMs in water electrolysis applications, and a representative PFSA polymer is shown in Figure 2. This type of polymer is obtained through the copolymerization of tetrafluoroethylene and perfluoroethylene with a sulfonic acid group. Due to their perfluorinated structure, PFSA materials have high chemical stability.
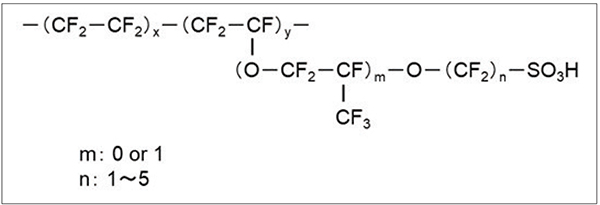
FIGURE 2. A molecular structure of a representative perfluorinated sulfonic acid (PFSA) polymer is shown
Furthermore, the hydrophobic main chain (with a polytetrafluoro ethylene structure), and the hydrophilic side chain (with a sulfonic acid group), form a phase-separated structure, creating an ion cluster where sulfonic acid groups are accumulated [6]. A structure of ion clusters has been proposed to be linked with narrow channels by Gierke [7], which is a probable reason for the high proton conductivity of perfluorinated sulfonic acid polymers (Figure 3) [6–8].
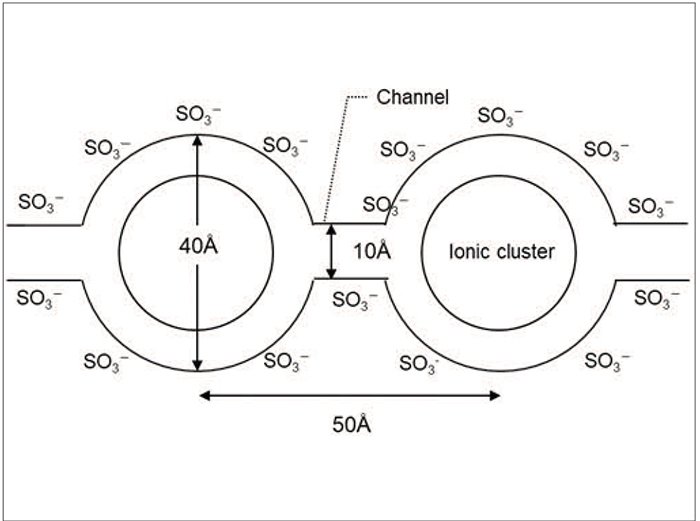
FIGURE 3. Perfluorinated sulfonic-acid polymers exhibit high proton conductivity, which is thought to be caused by the formation of ion clusters [7], as shown here
Proton conductivity is known to depend on the number of sulfonic acid groups. The ion-exchange capacity (IEC; typical units: meq/g) is an index that represents the amount of sulfonic acid (meq) per unit weight (g) of a polymer. For a PFSA polymer synthesized by changing the copolymerization ratio, the membrane resistance, as well as IEC, can be tuned. The higher the IEC is, the lower the distance between ion clusters is, which is a probable reason that polymers with high IEC values also exhibit high proton conductivity [ 8, 9]. In addition, the IEC and molecular structure of the polymer also affect hydrogen permeability and mechanical strength. Perfluorinated sulfonic acid polymer has been used in various applications, such as chlor-alkali electrolysis and fuel cells because of these characteristics.
PEM design strategy
The following paragraphs describe several design techniques for PEMs that can help realize the four required characteristics for efficient water electrolysis.
A PEM consists of polymer, reinforcing materials and additives. One method to improve electrolysis efficiency is to use a polymer with a high IEC. Reducing membrane thickness is also an effective method to reduce membrane resistance, but because hydrogen-shielding ability decreases, there is a trade-off between improving electrolysis efficiency and safety. A technique to minimize the impact of this compromise is the use of a GRC, which is a catalyst additive that converts the hydrogen that has permeated through a PEM into water via a reaction with oxygen. This technique allows for a dramatic reduction in the concentration of hydrogen in the anode, enabling differential high-pressure water electrolysis under safe conditions. Platinum is known as an effective GRC [10]. By applying a GRC and membrane thinning, it is possible to improve hydrogen shielding ability and electrolysis efficiency.
There is also a need to enhance the chemical stability of PEMs to withstand the presence of hydroxyl radicals generated during water electrolysis. A radical scavenger is a chemical species as an additive to reduce the amount of hydroxyl radicals by converting the radical into a chemically inert compound, which can suppress the decomposition of polymer. In fuel cells, inorganic compounds, such as cerium, manganese, chromium, cobalt and aluminum have been realized to act an effective radical scavenger, although some compounds have a problem of leaching out during power generation [11].
PEMs typically expand when hydrated. In-plane expansion can not only worsen the yield of membrane production and subsequent processes, but is also believed to impact electrolysis performance. Reinforcement can be applied to suppress the rate of dimensional change in the in-plane direction. Polytetrafluoroethylene (PTFE) has been used as a reinforcement material, enabling dramatic reductions in the rate of dimensional change.
As described previously, PEMs can be improved with a combination of the proper polymers, additives and reinforcing materials, along with meticulous attention to design. However, trade-offs and safety should be considered when optimizing the materials to achieve the target performance.
Further progress in PEM design technology is required to balance the four required characteristics for effective water electrolysis discussed previously — efficiency, durability, safety and compatibility. Additionally, the performance of PEMs is greatly influenced by other factors, such as the catalyst and catalyst-dispersion ionomers, GDL, flow field and heat control. ■
Acknowledgement
Images provided by authors
For more information, please contact Shunsuke Tsukuemoto at shunsuke.tsukuemoto@agc.com
References
1. K. Ayers, N. Danilovic, R. Ouimet, M. Carmo, B. Pivovar, and M. Bornstein, Annu. Rev. Chem. Biomol. Eng.,10, 219 2019.
2. S. Hayabe, H. Okada, and T. Sawada, J. Fuel Cell Sci. Technol., 23, 30, 2024.
3. S. Hayabe and K. Sumikura, J. Fuel Cell Sci. Technol., 20, 36, 2021.
4. M. Nur, I. Salehmin, T. Husaini, J. Goh, and A. B. Sulong, Energy Convers. Manag., 268, 115985, 2022.
5. S. Hayabe, K. Sumikura, and M. Ohkura, Chem. Eng., 40–42, 2021.
6. K. A. Mauritz and R. B. Moore, Chem. Rev., 104, 4535, 2004.
7. T. D. Gierke, G. E. Munn, and F. C. Wilson, J. Polym. Sci.: Polym. Phys. Ed., 19, 1687, 1981.
8. S. Hayabe, T. Okuyama, K. Sumikura, and T. Nishio, Hydrogen Production Technologies by Water Electrolysis – Basics and state-of-the-art technologies of various water electrolysis methods, and trends of global hydrogen policies, Tokyo: CMC Research, Ltd., 69-74, 2023.
9. A. Kusoglu and A. Z. Weber, Chem. Rev., 117, 987, 2017.
10. C. Klose, P. Trinke, T. Böhm, B. Bensmann, S. Vierrath, R. Hanke-Rauschenbach, and S. Thiele, J. Electrochem. Soc., 165, F127, 2018.
11. Z. Rui and J. Liu, Prog. Nat. Sci.: Mater. Int., 30, 732, 2020.
Authors
 Yusuke Chiba is a researcher at AGC, Inc.’s chemicals company. Working on the development of ion-exchange membranes since 2023, his responsibilities include designing ion-exchange membrane technologies and evaluating their electrolysis performance. He holds a Ph.D. in engineering from Kyoto University, where he studied supramolecular chemistry and organic synthesis.
Yusuke Chiba is a researcher at AGC, Inc.’s chemicals company. Working on the development of ion-exchange membranes since 2023, his responsibilities include designing ion-exchange membrane technologies and evaluating their electrolysis performance. He holds a Ph.D. in engineering from Kyoto University, where he studied supramolecular chemistry and organic synthesis.
 Shintaro Hayabe is a researcher at AGC, Inc.’s chemicals company, where he has worked since 2017. He graduated from Keio University with a M.S. degree in engineering. He has been working with materials related to ion-exchange membranes for about seven years. His responsibilities include the development of new ion-exchange membrane technologies and the evaluation of their actual performance.
Shintaro Hayabe is a researcher at AGC, Inc.’s chemicals company, where he has worked since 2017. He graduated from Keio University with a M.S. degree in engineering. He has been working with materials related to ion-exchange membranes for about seven years. His responsibilities include the development of new ion-exchange membrane technologies and the evaluation of their actual performance.
 Toshiaki Sawada is a group leader in the Research & Development Division of AGC, Inc.’s chemicals company, where he has worked since 2023.He graduated from Kyoto University with a M.S. degree in engineering. He has been working at AGC since 2007 and has been involved in the development of fuel cell materials and the fluoropolymer film products. His responsibilities include determining the direction of new ion-exchange membrane development.
Toshiaki Sawada is a group leader in the Research & Development Division of AGC, Inc.’s chemicals company, where he has worked since 2023.He graduated from Kyoto University with a M.S. degree in engineering. He has been working at AGC since 2007 and has been involved in the development of fuel cell materials and the fluoropolymer film products. His responsibilities include determining the direction of new ion-exchange membrane development.
 Shunsuke Tsukuemoto is a sales and marketing manager at AGC Chemicals Americas, Inc. (AGCCA) and has worked there since 2023. In his current role, he is responsible for sales and marketing of ion-exchange membranes and ionomer solutions. He holds a B.S. degree in economics from Keio University.
Shunsuke Tsukuemoto is a sales and marketing manager at AGC Chemicals Americas, Inc. (AGCCA) and has worked there since 2023. In his current role, he is responsible for sales and marketing of ion-exchange membranes and ionomer solutions. He holds a B.S. degree in economics from Keio University.
 Will Salem is a technical manager at AGC Chemicals Americas, Inc. (AGCCA) and has worked there since 2018. In his current role, he is responsible for AGCCA’s technical service and product development of ion-exchange technologies. He holds a B.S. in materials science and engineering from Pennsylvania State University.
Will Salem is a technical manager at AGC Chemicals Americas, Inc. (AGCCA) and has worked there since 2018. In his current role, he is responsible for AGCCA’s technical service and product development of ion-exchange technologies. He holds a B.S. in materials science and engineering from Pennsylvania State University.