Mechanical processes can offer an environmentally friendly and safer option to chemical water treatment
Non-chemical methods for conditioning water for use in cooling towers and boilers have been investigated, marketed and installed for over 100 years. In light of the recent incentives to “go green,” these methods offer engineers, operators and owners the option of replacing corrosive, toxic chemicals with physical or mechanical processes that claim to produce the same or superior results to those obtained by traditional chemical treatment methods — often without many of the attendant environmental, health and safety concerns.
Because of the growing interest in conserving water, reducing discharge of chemicals into the environment, limiting exposure of workers to hazardous chemicals, and the ever-present need to save energy, engineers are once again reviewing the benefits of non-chemical water treatment methods. A review of the literature along with many years of experience indicates that some of these methods produce results as promised by the manufacturer, whereas others fall short of this goal.
The array of non-chemical water treatment equipment is impressive. And the assertions for the benefits derived from using these devices are equally impressive. A simple, but not all-inclusive, list of equipment types is as follows:
• Magnetic
• Electrostatic
• Ultrasonic
• Galvanic or cathodic
• Electro-chemical
• Electro-deposition
• Electro-deionization (EDI)
• Membrane separation
• Ozone
• Ultraviolet
The claims made for these devices include the prevention of scale in boilers and heat exchangers and the control of corrosion on steel, copper, galvanized steel and other alloys. In the case of cooling tower operation, certain non-chemical methods are claimed to reduce bacterial growth that produces biofilms on system components.
This article offers an unbiased presentation and discussion of the claims made for the various non-chemical water treatment methods, a scientific explanation for how they work (or don’t work), and recommendations for the selection and use of non-chemical water-treatment equipment in utility and process applications.
Magnetic field devices
Magnetic fields created by permanent and electromagnets are incorporated into many water conditioning devices. As early as 1873, A.T. Hay was issued a patent for the use of an electromagnetic field to prevent scale in steam locomotives. Since then, permanent magnets have been mounted inside pipe sections and reaction chambers or clamped to the outside of pipe runs to cause the water to be conditioned as it flows through the magnetic field.
More recently, induction coils (solenoids) producing anywhere from 0.060 to 100-kHz electromagnetic fields have been used to condition water. The coil is wrapped around a length of PVC or stainless-steel pipe to form a reaction chamber as shown in Figure 1. The strength of the magnetic field is proportional to the current flowing through the coil and the number of turns of the wire.
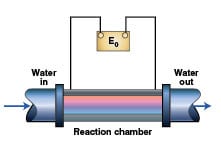
The manufacturers of this equipment claim that it controls scale in heat exchangers by modifying the surface charge on particulate matter in the water. This allows scale-forming ions, such as calcium and carbonate, to react on the surface of the particulate or colloidal matter resulting in the formation of calcium carbonate powder that preferentially settles out in the tower basin or is removed by a sidestream separator instead of forming hard, calcite scale in the heat exchanger.
The magnetic field is also said to control bacteria populations by electroporation. By this method, bacteria cell walls are ruptured by exposure to electromagnetic fields that vary in strength over time.
A major disagreement and debate exists in the literature and amongst consultants on the efficacy of magnetic water-conditioning equipment. Most of the research on this subject has been conducted in the former Soviet Union with very favorable results. These devices are reported to be used with great success and economic advantage.
The results of independent investigations performed in the U.S., however, are almost universally negative. In a well-publicized paper presented in 1958 at the AWWA Annual Conference by Eliassen, Skrinde and Davis of the Massachusetts Institute of Technology [1], the conclusions reached by these researchers indicated that magnetic water conditioners produced no measurable or permanent change in the physical or chemical properties of water in terms of the ability of the magnetic field to alter scale formation or inhibit corrosion. The effect, if any, of the magnetic field would be limited to the residence time inside the reaction chamber. The magnetic field does not impart any permanent alteration of the chemical properties of the water.
This is not to say that electromagnetic devices do not have their ardent supporters. Very favorable results have been reported by investigators, but these are generally based on empirical evidence, visual inspections and testimonials from satisfied users. Typically, the research is conducted using protocols established by the manufacturers of the equipment who have a financial interest in the outcome of the evaluation. Clearly, further research by an independent, unbiased organization is required to verify and substantiate the claims made by the marketers of magnetic water conditioners.
Electrostatic devices
Another class of non-chemical water conditioning devices focuses on passing the water through an electrostatic charge. These are designed with a positively charged insulated, central electrode that is inserted into the center of a grounded cylindrical casing, which serves as the negative electrode. The application of high voltage on the central electrode produces an electrostatic charge across the annular space between the electrodes. The water is conditioned as it flows rapidly through the electrostatic field.
These units operate at 110–120 V (60 Hz), but typically draw a very low current of about 0.1 A. This suggests that very little work is done since the power requirement is only 1 to 2 W.
These devices are said to work by virtue of the water molecules being rearranged into an orderly array between the electrodes. This causes the scale-forming ions, such as calcium and magnesium, to be surrounded by a “cloud of water molecules,” thus preventing scale formation. The device is also claimed to remove old scale deposits by promoting increased solubility through reduced surface tension of the water.
In addition to scale prevention, the manufacturers of electrostatic water-conditioning equipment claim that bacteria are controlled by disruption of the charged surfaces of the cell wall. This interferes with the organisms’ ability to absorb nutrition and reproduce.
Like with the magnetic water conditioners, little independent evidence exists in the U.S. to support the claims made by the electrostatic equipment manufacturers beyond testimonials and subjective visual inspections of plant equipment. Several of these devices were actively marketed in the 1970s by reputable industrial-equipment manufacturers, but have since been discontinued.
Ultrasonic water treatment
Ultrasonic water treatment is primarily targeted at preventing or controlling bacterial growth in water-using systems. Sound waves outside the range of human hearing are produced by a low power, high-frequency generator inside a reaction chamber. The microorganisms are destroyed by the ultrasonic wave energy that causes fatal changes inside the bacteria cells.
The medical literature indicates that high-energy ultrasonic generators have been shown to be effective in killing bacterial and viral organisms. However, this requires high power and a prolonged contact time. Sizing a unit for a typical industrial cooling tower that is capable of providing sufficient power (kilowatts) at the design flowrate is a challenge.
Notwithstanding the size of the unit, the antibacterial properties of the ultrasonic device are limited to killing organisms that are free-floating in the water (planktonic). The ultrasonic waves produce no residual effect and are, therefore, incapable of controlling or limiting the growth of biofilms (sessile organisms) and algae.
Electrochemical methods
Several classes of water treatment equipment are designed around the fundamental scientific principles of electrochemistry. These rely on an anode (+), a cathode (-), a current path, and an electrolyte (in this case water). A simple illustration of an anode/cathode cell is depicted in Figure 2.
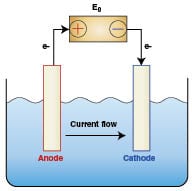
Sacrificial anodes and cathodic protection: Corrosion is considered to be an electrochemical process whereby current flows from the anode to the cathode. A chemical reaction (oxidation) occurs at the anode causing metal to be dissolved into the water; that is, corrosion occurs at the anode. A complementary chemical reaction (reduction) occurs at the cathode. No corrosion occurs at the cathode as it is “protected” by the current that flows onto the metal surface from the anode.
If two dissimilar metals are coupled together in an anode/cathode cell, the less noble or less stable metal will become the anode. The anode is sacrificed thereby protecting the more noble metal, which functions as the cathode. Thus, if zinc is coupled with steel, for example, the zinc anode will be consumed as current flows from the zinc onto the steel. The higher the corrosion current, the faster the anode will be consumed. Galvanized steel is thus protected by virtue of the 3–4 mil zinc coating being slowly sacrificed to protect the underlying steel.
It is possible to enhance the corrosion protection by impressing a d.c. current from either a battery or rectifier. In this case, the impressed current flows from the anode through the water and onto the cathode. Sufficient overvoltage must be applied to establish a current density on the metal surface that is sufficient to maintain passivation of the metal to be protected. The negative terminal of the rectifier must be connected to the structure to be protected, otherwise it will be established as the anode (+) and corrode. This corrosion control method is used to protect buried and underwater structures, gas pipe lines, ship hulls and water towers throughout the world.
Electrolysis: Direct current (d.c.) electricity is used to produce oxidation/reduction chemical reactions in a variety of chemical processes. Chlorine, caustic soda, aluminum, magnesium and copper are made or refined industrially in large electrochemical cells.
On a smaller scale, electrolysis can be used to generate chlorine and bromine on site from an electrolytic cell that uses sodium chloride salt or a mixture of sodium chloride and sodium bromide salts as the feedstock. In this case, a prepared 3–4% brine solution is used to produce chlorine at the anode with hydrogen and hydroxide produced at the cathode. The chlorine is mixed with water to produce a 0.4–0.8% sodium hypochlorite solution that is either stored in a holding tank for future use or dosed directly from the generator into the tower. The hydrogen is vented to the atmosphere.
Onsite electrochemical chlorine generators eliminate the need to store gaseous chlorine, which is a regulated substance, and 12% liquid chlorine, which is corrosive and tends to slowly decompose during storage.
Electro-deposition: One of the fundamental goals in cooling tower operation is to prevent scale deposition on heat transfer surfaces due to the precipitation of sparingly soluble salts of calcium carbonate, magnesium hydroxide and silica. This is traditionally accomplished by the judicious control of tower bleed to limit the cycles of concentration. Chemical scale inhibitors are also routinely used to enhance the solubility of these scale-forming salts.
More recently, a new method of scale control has been introduced that removes these scale forming impurities by the electrochemical deposition of calcium and magnesium (and other) salts at the cathode of an electrochemical cell. Direct current is applied to the cell at a rate sufficient to drive the precipitation reactions at the cathode. These devices are electro-synthesis cells that produce a buildup of calcium hydroxide, magnesium hydroxide and other salts at the cathode. These insoluble salts are then removed to allow the continued flow of current from anode to cathode.
The driving force for the electrochemical cell reaction is determined by the voltage applied between the electrodes. The total voltage is determined by the theoretical voltage, which is the sum of the anode and cathode half-cell voltages; the overvoltage required to achieve the desired production level; and the electrolyte resistivity (water resistivity). This determines the total cell voltage required to drive the reaction.
The current requirement can be estimated from a formula worked out by Faraday that expresses the quantity of electric charge required to produce the desired yield of precipitated salt. This is the product of the electric current and the length of time it flows through the cell. The estimated power requirement is determined by multiplying the voltage times the current flow to yield the kilowatt-hour per kilogram of salt produced. Because of the high electrical resistance of water, the power cost for this process can be significant over traditional chemical treatment methods.
Other factors that should be reviewed with this process include the cost for equipment maintenance, solids disposal and electrode replacement. As with all electrochemical processes, the selection and durability of the anode is important.
Microbiological control
Ozone: Ozone is second only to fluorine as an oxidizing agent. As such, ozone functions as a very strong oxidizing biocide in cooling towers and drinking water systems. It has been marketed as an alternative to other oxidizing biocides, such as chlorine and bromine, for bacteria and algae control in cooling towers since 1970.
Ozone is produced in a corona discharge generator by passing a stream of dry air through an electric arc to yield O3. The generators come in various output capacities depending on the rated capacity of the cooling tower and microbiological demand. Typically, 0.5 to 1.0 lb of ozone per 100 tons of air conditioning is employed. The power consumption is about 15 kWh per pound of ozone produced.
Most experts agree that ozone is very effective in controlling microbiological growths in cooling towers. However, additional claims by ozone proponents that it conserves water, prevents scale deposition and mitigates corrosion are in dispute. Some ozone programs have been applied with no tower bleed resulting in the deposition of a white sludge in the tower basin and low flow areas of the system. Because it is such a strong oxidizing agent, ozone tends to attack materials of construction if overly applied or poorly controlled.
Ultraviolet light: Ultraviolet (UV) lamps produce light with a wavelength of 254 nm. When bacteria are exposed to UV radiation, the organisms are rendered unable to reproduce and thus considered dead. This process is most effective in water that is relatively clean and pure to minimize the absorption of light by suspended solids and other debris.
The UV dosage required to destroy microorganisms is measured in microwatt-seconds per centimeter squared (µWs/cm2). Depending on the organism (bacteria, yeast, mold, viruses, algae), this can vary from 2,500 to over 26,000 µWs/cm2.
Ultraviolet light is only lethal during the time that the organism is exposed directly to the light. It produces no residual effect in the water and therefore, does not kill biofilms that form on surfaces not exposed to the UV radiation.
Membrane separation
Another class of non-chemical water treatment methods that have come into their own in the last 30 years is reverse osmosis (RO) and electro-deionization (EDI). These processes remove over 99% of the dissolved solids present in the raw feedwater to produce a purified water stream.
Reverse osmosis (RO): RO utilizes a pressure differential across a semipermeable membrane to reject dissolved salts at the membrane surface while allowing the purified water to permeate through the pores of the membrane. These membrane separators have been fabricated in a variety of configurations including spiral wound and hollow fiber modules. (For more information, see: Strategies for Water Reuse, CE, September 2009, pp. 34–39.)
The RO process produces a concentrated brine stream that is typically 25% of the feedwater flow. As such, reverse osmosis has a lower recovery rate of 75% as compared to ion exchange. Unless a use for the RO reject is found, reverse osmosis will consume more fresh water than ion exchange.
On the positive side, RO is a continuous process that doesn’t require the use of regeneration chemicals like concentrated acid and caustic soda that are used in the batch regeneration of ion exchange demineralizers.
Electro-deionization (EDI): This process is similar to RO in that it utilizes ion exchange membranes and resins to separate the feedwater into a purified water stream and a concentrated brine stream. Instead of pressure differential, however, this is done in conjunction with an electric field produced by the potential difference between an anode (+) and cathode (–). The potential difference between the electrodes creates the driving force across the membrane. Positively charged ions selectively pass through the membrane and are attracted to the cathode. Negatively charged ions are separated by the membrane and move toward the anode. The result is a final product stream of de-ionized water as illustrated in Figure 3.
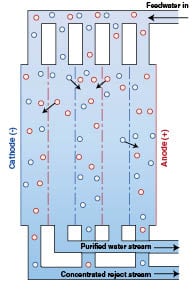
Reverse osmosis and electro-deionization are used in many applications to replace more traditional ion-exchange processes. When used in place of ion exchange demineralizers, for example, the acid and caustic regeneration chemicals can be eliminated. This limits worker exposure to these chemicals, reduces the amount of acid and caustic discharged to waste, and eliminates the need to purchase, ship, store and handle corrosive chemicals.
Concluding remarks
The non-chemical water treatment methods discussed in this article share one thing in common. They utilize electric current in one fashion or another to condition water. Instead of buying, shipping, storing and feeding chemicals to prevent scale, mitigate corrosion, and control microbiological growths, these devices simply plug into the wall. This feature offers many benefits at a time when plants are seeking ways to decrease worker exposure to hazardous chemicals and reduce waste disposal costs.
However, as indicated, some of these devices make claims that are difficult to substantiate based on independent, unbiased, scientific evaluation. As expected, the manufacturers of this equipment offer testimonials and case studies to support their claims. Notwithstanding claims to the contrary, many cases have been reported where the equipment failed to perform as advertised, resulting in equipment damage and unscheduled downtime. For this reason, it is best to seek the advice of an unbiased, knowledgeable expert when considering the application of non-chemical water treatment methods.
The good news is that when properly applied, many of the non-chemical technologies discussed in this article help plants conserve water, reduce chemical consumption, minimize waste, and save energy. This is not only good for the environment, but good for business, too. ♦
Edited by Gerald Ondrey
Reference
1. Eliassen, R., Skrinde, R. T., Davis, W. B., Experimental Performance of ‘Miracle’ Water Conditioners, J.Am. Water Works Assoc., Vol. 50, No. 10, October 1958.
Author

William Harfst is president of Harfst and Associates, Inc., an independent water management consulting firm (P.O. Box 276, Crystal Lake, Ill. 60039; Phone: (815) 477-4559; Email: [email protected]; Website: http://www.harfstassociates.com). He has over 36 years of water treatment experience helping industrial, institutional, commercial and government clients select, apply and control water treatment programs for boiler, cooling and wastewater applications. He graduated from the University of Illinois with a B.S. in Chemistry cum laude in 1972 and went on to hold various technical and management positions with three major water treatment companies before starting his consulting practice in 1991. His current focus is on helping clients conserve water, reduce chemical consumption, minimize waste and save energy.