Amino acids are the basis for all proteins and are considered building blocks of life. Among them, lysine (biologically active in its L-stereoisomer form) is one of the essential amino acids not synthesized biologically in the body. In this context, lysine is typically used as a supplement in human food and animal feed.
L-lysine is commonly commercially produced as L-lysine monohydrochloride (L-lysine HCl), with purity higher than 98.5 wt.%, which corresponds to 78.8 wt.% of free lysine.
The process
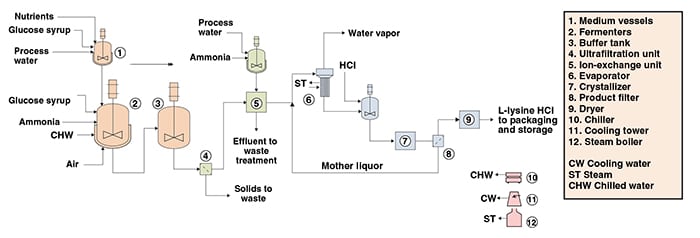
The following paragraphs, along with Figure 1, describe a conventional fermentation process for L-lysine HCl production. The process can be divided into three main parts: fermentation; product recovery; and product concentration, drying and packaging.
Fermentation. The culture media used in the batch and fed-batch phases of fermentation are prepared by mixing process water, glucose and nutrients. The fermentation step is performed in fed-batch mode and under aerobic conditions. In the batch phase, the microorganism seed is fed into the fermenters, which have been filled previously with the fermentation batch medium. After glucose exhaustion, the batch phase is finished and the fed-batch phase is started. During the fed-batch phase, glucose and nutrients are continuously supplied until the desired L-lysine concentration is achieved. At the end of the fermentation, the broth is sent to a buffer tank to provide a continuous flow to the downstream process steps.
Product recovery. The fermentation broth is sent to an ultrafiltration system for the removal of cell debris and other suspended solids. Subsequently, the liquor from ultrafiltration is fed to ion-exchange columns, where L-lysine is selectively adsorbed. The adsorbed L-lysine is eluted from the ion-exchange resins by washing with an aqueous ammonia solution.
Product concentration, drying and packaging. The L-lysine eluted from the ion-exchange columns is mixed with mother liquor from the product-filtration step and concentrated by evaporation. The concentrated lysine solution is acidified with hydrochloric acid, and free L-lysine is converted to L-lysine HCl. The L-lysine HCl solution is then sent to the crystallizer, and lysine salt is filtered. The mother liquor is recycled to the evaporator and the wet cake is conveyed to dryers. Final dry L-lysine-HCl (98.5 wt.%) is obtained and sent to a packaging line before being stored in bags.
Economic performance
The total capital investment estimated for construction of a plant producing 100,000 metric tons per year of L-lysine HCl in the U.S. is about $350 million. The capital investment includes total fixed capital, working capital and additional capital requirements. Other assumptions considered are:
Period of the analysis is second quarter of 2015. The plant includes installations required to provide a total storage capacity of 20 days for raw materials consumed and product generated.
Global perspective
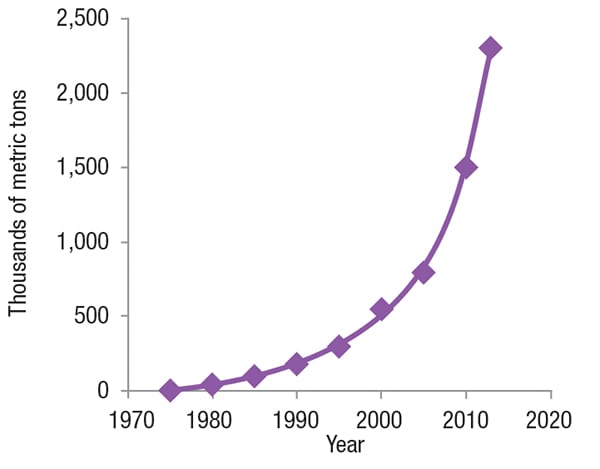
Worldwide production of white meat (for example, poultry and pork) has significantly increased over the past forty years. The growth has led to a much higher demand for L-lysine. Figure 2 illustrates the growth in L-lysine production over the past several decades.
Edited by Scott Jenkins
Editor’s Note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.