In hygienic process operations, proper system construction and fabrication has the greatest influence on end-product quality and the success of process validation. Even well-designed hygienic systems can be compromised by leaching, poor internal finishes and mishandled material sourcing and other fabrication issues. All can lead to contamination and bacterial growth in the system. This one-page reference provides information on key quality-assurance procedures for the fabrication and assembly of hygienic systems that will help prevent contamination and bacteria growth during operation.
Contamination can come from unlikely sources, such as process media reacting with rubber over time, causing carbon to be extracted from rubber parts. Pipes can have improper finishes or poor welds, creating safe harbors for bacteria to grow. Following proper procedures can minimize potential issues.
Setup considerations
The following are considerations for each stage of the hygienic process build, from sourcing to assembly.
Parts and materials purchasing. From raw metal suppliers to shop welders, materials must be handled properly, and procedures should create individual responsibilities for verifying proper handling at every step. Important information includes the source of the metal materials and what requirements those materials need to meet. Determine what documentation (for example, proof of chemical composition) is needed from each supplier and conduct a review of the orders to verify that they meet the requirements for materials of construction.
Material handling. During sourcing of process components, it is important to know how equipment parts and materials are handled and shipped from the supplier. For example, what do parts touch during shipping and how are they handled during receiving? Once the parts arrive, there should also be an organized way to identify parts at the facility. Also, a procedure is needed to verify the mill test report (MTR) review, logging and tracking. What are the conditions under which parts are stored and who is responsible?
Fabrication and assembly. A procedure should be in place to approve parts and materials for use, as well as to ensure that parts are fabricated, welded and tested correctly. A third-party Level-2 certified welding inspector (CWI) should inspecting welds in the process equipment.
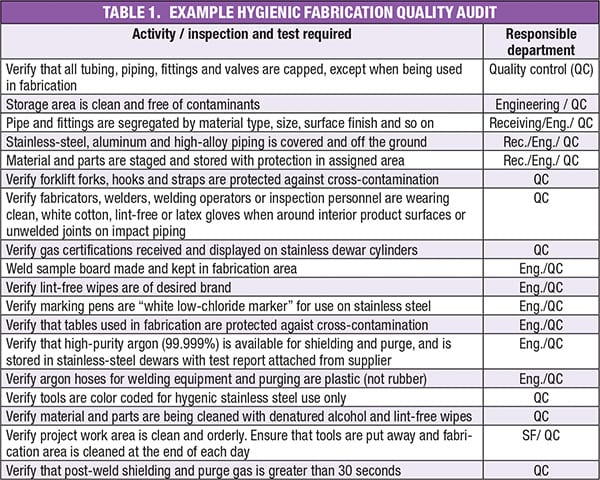
Fabrication quality audit
Conducting a fabrication quality audit can help ensure that necessary quality assurance activities are completed according to the correct procedures. An example audit list, showing activities, inspections and tests, is presented in Table 1. Proper storage methods are included for verification. Also, the standards (not shown here) against which the activities should be verified are listed next to each item with a personal sign-off to verify proper procedures have been followed throughout. Validation sheets matching each procedure ensures the process was followed and documented. The following are areas that would be covered by hygienic quality assurance audit:
Weld documentation. This includes the welder’s name, when the weld was performed, and what materials were used. Also included is which weld procedure was performed and whether it was the proper procedure. Other questions include: how much oxygen was used during the welding? Who inspected the weld and when?
Assembly documentation. This includes, for example, the type of gaskets used, documentation showing where they were used and the date installed, as well as an audit of all joints, verifying that the correct gaskets are present and correctly installed.
Proper fabrication validation. As an example, hygienic systems are designed to eliminate dead legs during fabrication. Questions that should be asked include the following: is everything on the assembled system properly sloped? Were pockets created during fabrication? Did all piping get processed to the correct internal finish? Which requirement are you verifying against?
Editor’s note: Content for this column was provided by Jeff Koenigs, principal project manager and professional engineer, EPIC Process Systems (www.epicmodularprocess.com)