Any corrosion process in which microorganisms initiate, facilitate or accelerate corrosive chemical reactions is termed microbially influenced corrosion (MIC). MIC occurs when the chemical, microbiological and physical conditions allow the active growth of biofilms containing specific types of microbes on the surface of equipment, and the resulting growth fosters the electrochemical reactions that lead to corrosion.
Mechanisms and morphology
Microbial action has been identified as a contributor to rapid corrosion of metals and alloys exposed to a variety of media, including soil, seawater, distilled water, freshwater, crude oil, hydrocarbon fuels, process chemicals and sewage. MIC does not produce corrosion morphology that is distinct from conventional corrosion. MIC often results in pitting, crevice, under-deposit, and galvanic corrosion, as well as dealloying. The presence of water is required, although only a small amount is necessary. Proteins and metabolites secreted from microbes, including sulfide, organic acids and other compounds are often important factors in MIC.
The mechanisms of MIC are not completely understood, because it is generally very difficult to connect the phenomena to a single biochemical reaction or specific microbial species or group. Severe MIC in a natural environment is always caused by microbial communities with many different types of species. Metabolic products of the bacteria alter the interface chemistry, resulting in gradients of dissolved oxygen and pH. These gradients can lead to localized corrosion.
Biofilms
 Traditional understanding of MIC involves the formation of biofilms that provide a niche for corrosive microbes to grow and proliferate. Biofilms generally consist mostly of water and extracellular polymeric substances (EPS), which are mostly polysaccharides and proteins that act as structural materials for the biofilm, as well as other microbial metabolites, organic and inorganic molecules. Corrosion-relevant microbes attach to solid surfaces via EPS.
Traditional understanding of MIC involves the formation of biofilms that provide a niche for corrosive microbes to grow and proliferate. Biofilms generally consist mostly of water and extracellular polymeric substances (EPS), which are mostly polysaccharides and proteins that act as structural materials for the biofilm, as well as other microbial metabolites, organic and inorganic molecules. Corrosion-relevant microbes attach to solid surfaces via EPS.
Microorganisms live either in planktonic (free-floating) or sessile (attached) form. For corrosion, planktonic microorganisms are less dangerous. The number and types of microorganisms in the liquid phase of a hydrocarbon, for example, are not necessarily indicative of the potential for MIC, since the sessile microbes have the greatest influence on localized corrosion. Molecular microbiological methods, such as DNA- or enzyme-based test methods, provide important information about biofilm communities.
In aqueous environments, when microbes that prefer to exist in the sessile form adhere to solid surfaces, biofilms form. The metal degradation that occurs with MIC takes place underneath the biofilm. Gradients of microorganisms, oxygen concentrations and pH values exist within biofilms, and these gradients, along with local conditions, form anodic and cathodic sites on metal surfaces. If uncontrolled, localized corrosion occurs, leading to pinholes and leaks.
Bacteria species types
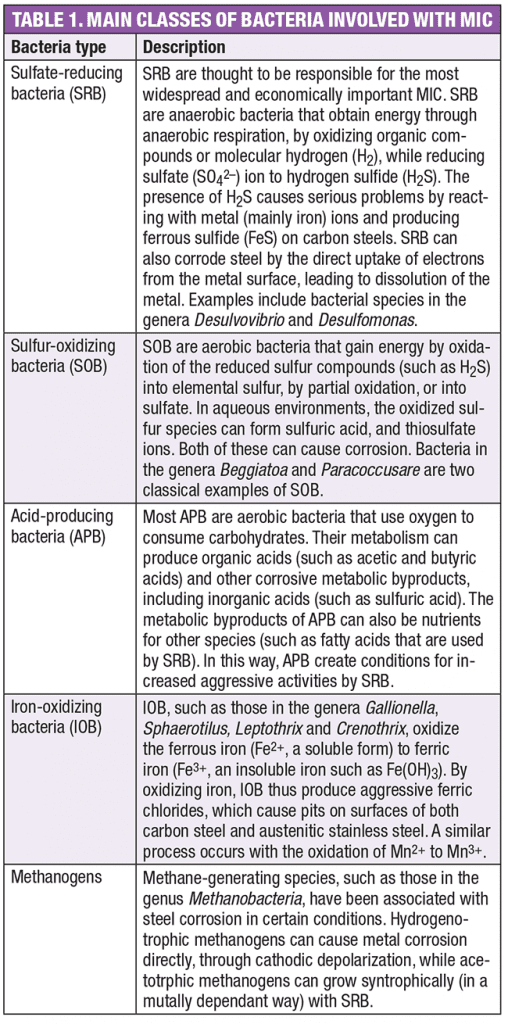
MIC typically takes place in the presence of a community of multiple types of microorganisms. Depending on the environment, these microbes may include sulfate-reducing bacteria (SRB), acid-producing bacteria (APB), metal-oxidizing bacteria, metal-reducing bacteria (MRB) and methanogens. Table 1 provides some information about the types of bacteria that are frequently associated with MIC.
Department Editor: Scott Jenkins
References
1. Telegdi, Judit, Shaban, Abdul, and Trif, Laszlo. Microbially Influenced Corrosion (MIC), appearing in “Trends in Oil and Gas Corrosion Research and Technologies,” Woodhead Publishing, pp. 191–212, 2017.
2. Kip, Nardy, and van Veen, Johannes. The dual role of microbes in corrosion, ISME Journal, Vol. 9 (3), pp. 542–551, 2015.
3. Riggs-Larseh, K. A Closer Look at Microbially Influenced Corrosion, Materials Performance, May 2020.
4. Webcor Corrosion Consulting Services, The Corrosion Clinic online resource, URL: www.corrosionclinic.com, accessed March 2021.