In Sweden, incineration of household waste in waste-to-energy (WtE) plants is common, and generates around 250,000 metric tons (m.t.) of flyash every year. The rest of Europe accounts for around ten times that amount. Most of this waste is landfilled, but the ash often contains significant amounts of valuable metals, such as zinc. A process to extract such metals has been developed by researchers at Chalmers University of Technology (Gothenburg, Sweden; www.chalmers.se), and is described in a recent issue of Waste Management.
The Chalmers process is much simpler than the one developed in the 1990s at the Karlsruhe University of Technology (KIT; Germany), and generates a zinc concentrate instead of a purified metal. The concentrate can then be further refined in existing industrial metals-processing lines.
In the Chalmers process (photos), an acid wash is used to release zinc and other metal ions from the ash. The zinc is recovered from the leachate as zinc hydroxide using chemical precipitation, which can then be further refined using metal industry processes to generate high-purity zinc metal. The leached flyash can be re-incinerated in order to destroy toxic dioxins. During the pilot study, 75–150 kg/h of flyash from a Swedish WtE plant was mixed with scrubber liquids from the same fluegas-treatment system in a continuously stirred vessel. The resulting slurry was dewatered in a vacuum belt filter. Hydroxide precipitation of the resulting leachate, followed by filtration of the formed crystals in a membrane filter press, produced a filter cake with up to 80 wt.% zinc hydroxide.
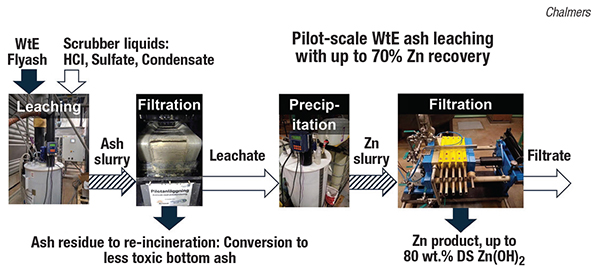
Renova AB and Babcock & Wilcox Vølund AB (both Gothenburg, Sweden) are now building an ash-washing facility with zinc recycling in Gothenburg — an investment that is estimated to save hundreds of thousands of euros every year for the municipally owned waste-management company.