The batch reactor is the workhorse of the fine chemical and pharmaceutical industries. Within it, many different unit operations are performed such as chemical reactions, bioreactions, crystallization, distillation and dissolution. Despite their significance to the industry, batch reactors have been controlled by the same underlying method for the past 50 years. This article discusses a relatively straightforward design change, constant flux control, which delivers compelling benefits to both temperature control and process monitoring.
These two aspects of batch reactor control, temperature control and process monitoring, are particularly important to manufacturing economics. Good temperature control is generally desirable and particularly so where product change is influenced by temperature (for example, crystallization, polymerization, chemical reactions and temperature sensitive materials). Conventional batch reactors tend to respond slowly to heat load changes. They also suffer from a variety of localized temperature deviations even when the bulk temperature appears satisfactory.
Product quality, yield and productivity can be optimized by employing ideal process methods. In many cases, however, the ideal process method is linked to factors that can vary from batch to batch. The only way to employ ideal process methods under these conditions is to link process control decisions (such as addition rate, reaction time and cooling rates) to realtime process analytical data. Although optical analytical instruments can be used for this purpose, they can present the user with significant problems (for example, cost, flexibility, reliability, fouling, calibration, ease of use, and area classification) when employed in the manufacturing environment.
New designs of the jacket for batch reactors, such as a variable-geometry heat-transfer surface, allow the user to regulate both jacket area and jacket temperature in realtime. This not only addresses long-standing temperature-control problems but also enables very sensitive heat-balance measurements to be made. This latter capability provides a simple and versatile process analytical technology (PAT) tool.
Temperature control
One of the most intractable problems of scale up is that of temperature control. Good temperature control in a batch reactor needs to take account of two factors. First, the temperature controller needs to be able to hold the bulk product temperature at the desired value and respond to changes in the heating or cooling load in a timely manner. Second, the wall temperature in the reactor needs to be maintained at levels that do not adversely affect the product.
A typical heating and cooling system for a conventional batch reactor is shown in Figure 1. The reactor body is surrounded by an outer jacket through which heat transfer fluid is circulated. On larger vessels, multiple injection points are used to improve distribution of the heat transfer fluid within the jacket.
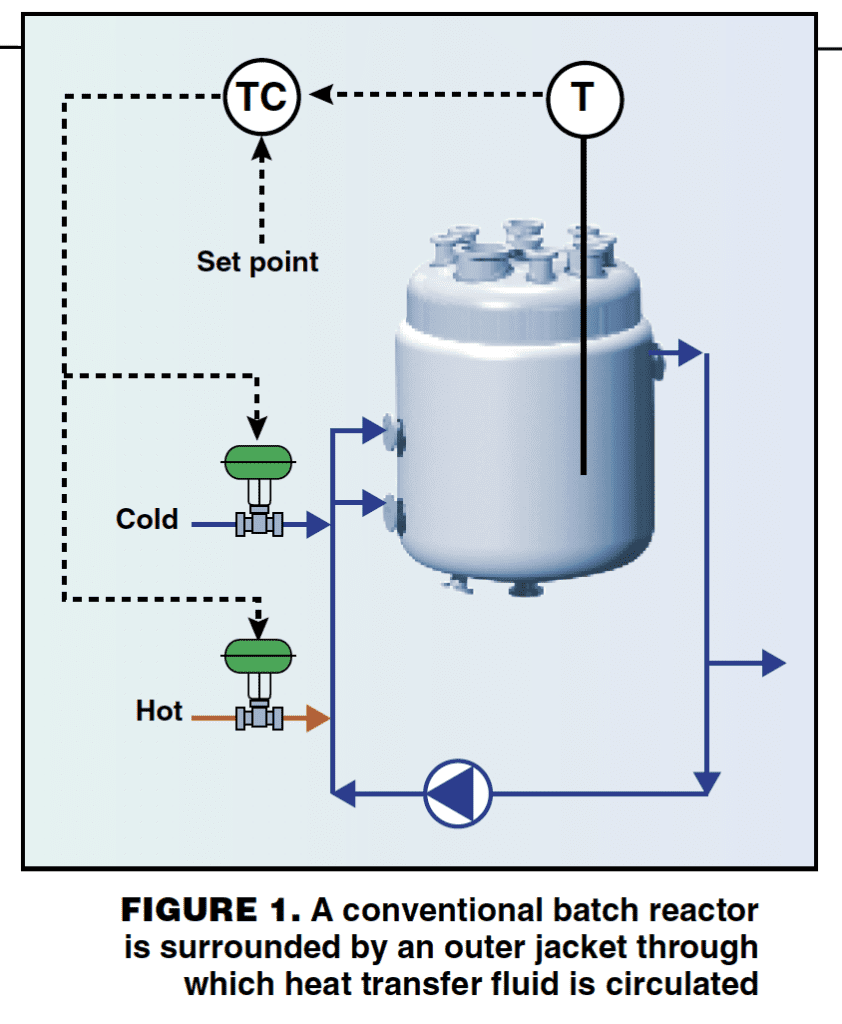
The cooling or heating power of the reactor is controlled by regulating the jacket temperature. This is achieved by injecting fresh hot or cold heat-transfer fluid into the circulation loop as required (an alternative arrangement uses external heat exchangers to raise or lower the heat transfer fluid temperature).
There are two common designs of reactor jacket (Figure 2). The most familiar is the one-piece jacket, which forms an outer chamber around the vessel. Heat transfer fluid is injected tangentially into the jacket at velocities in excess of 10 m/s. This promotes mixing and dispersion of heat transfer fluid within the jacket. The other common arrangement is the “half coil” jacket. The half coil jacket is fabricated as a series of pipes cut longitudinally and welded around the outside of the vessel. Heat transfer fluid travels in a plug flow manner through the channels.
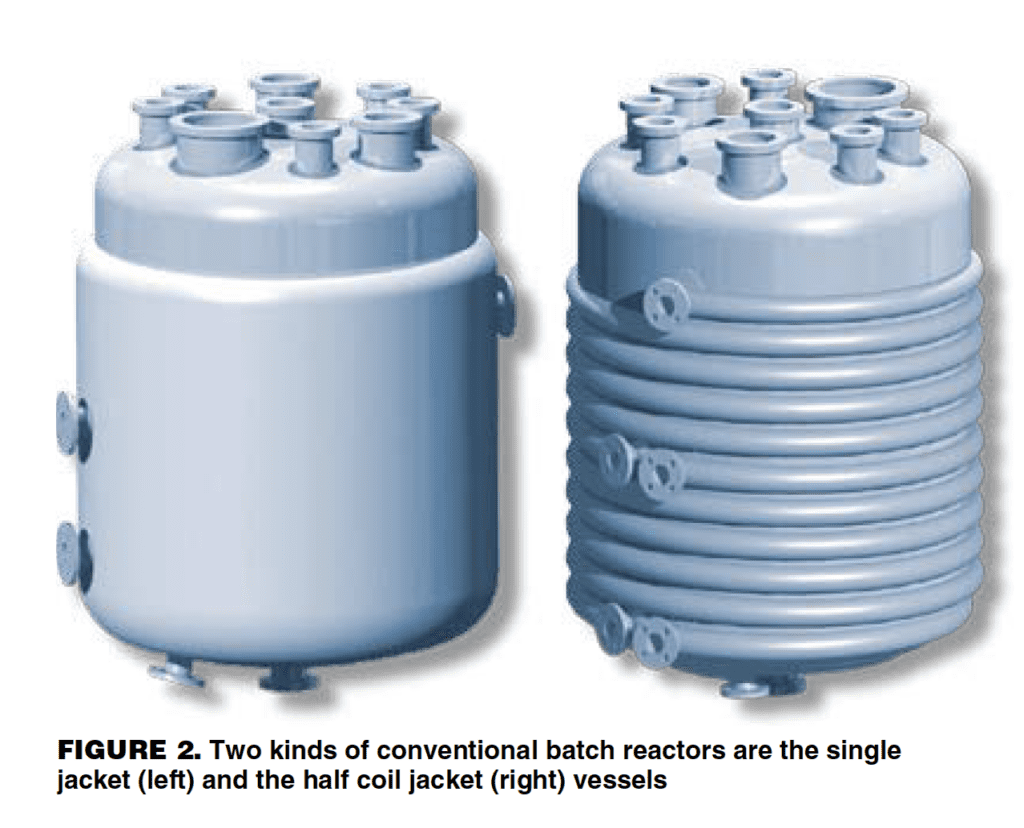
Apart from size, one of the most obvious differences between small and large reactors is relative heat-transfer area. A typical 5,000-L reactor, for example, has 30 cm2 of heat transfer surface per liter of product. By contrast, a 1-L lab reactor has more than 1,000 cm2/L. Thus, for the same thermal duty, the wall temperature of the 5,000-L reactor has to be 30 times hotter (or colder) than a 1-L vessel.
Operating reactor jackets at extreme temperatures can damage the product. The problem is further compounded by the fact that jacket temperature is a poor guide to the internal wall temperature. The graph in Figure 3 shows the relationship between vessel wall temperature and the process-side film coefficient under conditions where the jacket temperature and product temperature remain constant.
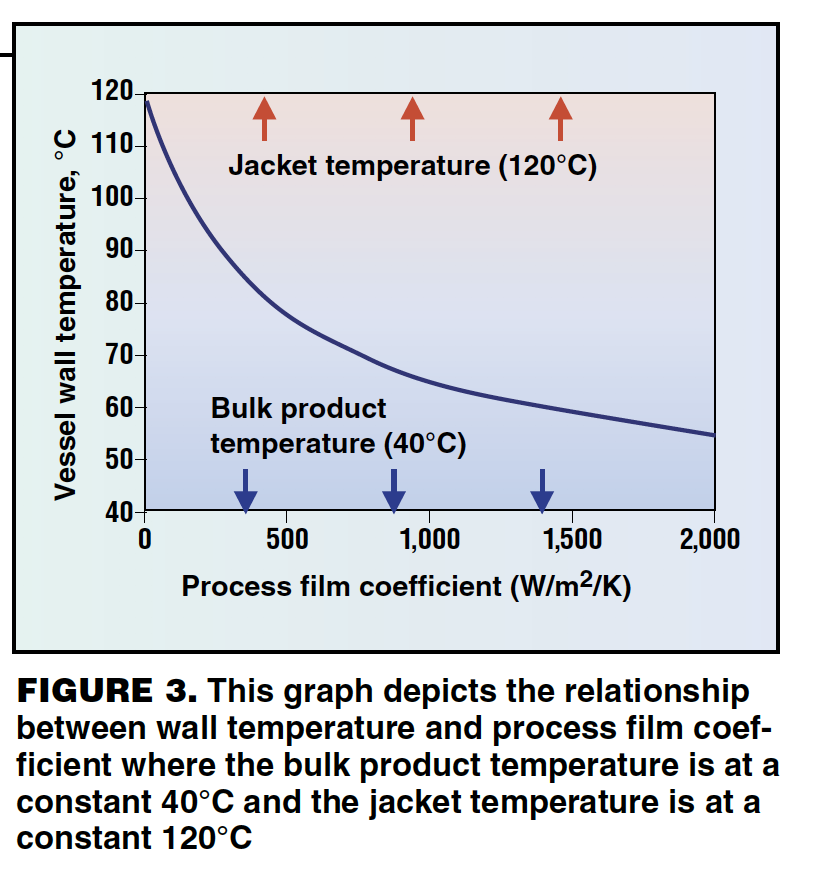
The process-side film coefficient is a measure of how easily heat can be transmitted between the product and the vessel wall. Factors that contribute to low film coefficients include high product viscosities, low thermal conductivity and poor agitation. In many operations, the film coefficient can change significantly during the process cycle with the result that a safe jacket temperature at one stage can cause surface burning or freezing at another. To protect the product from extreme wall temperatures, plant operators have to set jacket temperature limits. However, setting these to cope with low film coefficients at one part of the process means that the heat transfer capacity has to be restricted for the whole process cycle. This leads to unnecessarily long cycle times. A better solution is to monitor the wall temperature directly and use this to adjust the jacket temperature as necessary. Such measurements, however, are not practical in traditional batch reactors.
Restricting jacket temperature provides a measure of protection for the product. It does not eliminate hot or cold spots, however. In a typical industrial reactor, the control system can alter the fluid temperature entering the jacket by more than 150°C within a matter of seconds. While these fluctuations may have a relatively small effect on the average jacket temperature (and even less effect on the bulk product temperature), they can create severe, transient hot or cold spots at the jacket inlet points. The severity and frequency of these hot or cold spots is dependent on such factors as process temperature, heat transfer coefficient, proportional-integral-derivative (PID) control settings and prevailing conditions of the heat transfer fluid. This problem is an inherent weakness of using jacket temperature as the primary control parameter.
One other cause of hot (or cold) spots is the “dry wall” effect. Any heated (or cooled) wall surface inside the vessel, that is not covered by process fluid, will get significantly hotter (or colder as applicable) than the process temperature. Product which splashes onto this surface is exposed to more extreme heating or cooling conditions. The traditional solution to this problem is to use a split jacket. This is a heating and cooling jacket with upper and lower sections. By switching off the upper section of jacket, the user can restrict the heat transfer surface to a zone which is covered by process fluid. The split jacket concept, however, has the drawback that processes often have changing liquid levels. To cope with this, the split point for the lower jacket has to be set at a low level. This severely limits the heat transfer capacity.
Sluggish temperature control is a problem associated with large batch reactors and is responsible for slow and erratic temperature control of the product. This can be particularly undesirable for processes like crystallization where the required cooling load can change rapidly at nucleation. With conventional batch reactors, the turnover rate of heat transfer fluid in the jacket tends to fall as the vessel size increases. The turnover rate in a conventional 5,000-L reactor jacket, for example, is about 50 times slower than that of a 1-L reactor. This is the primary cause for temperature control problems on large systems. Historically, users of batch reactors have employed software solutions to compensate for slow jacket-turnover rates. This, however, means imposing more extreme temperature shifts in the heat transfer fluid entering the jacket. Although this can deliver modest improvements to response speed, it does so with the expense of creating more severe hot or cold spots within the jacket.
The problems of controlling temperature in batch reactors are related to hardware design, and for this reason they need a hardware solution. One solution is a reactor with a constant (heat) flux control system as shown in Figure 4.
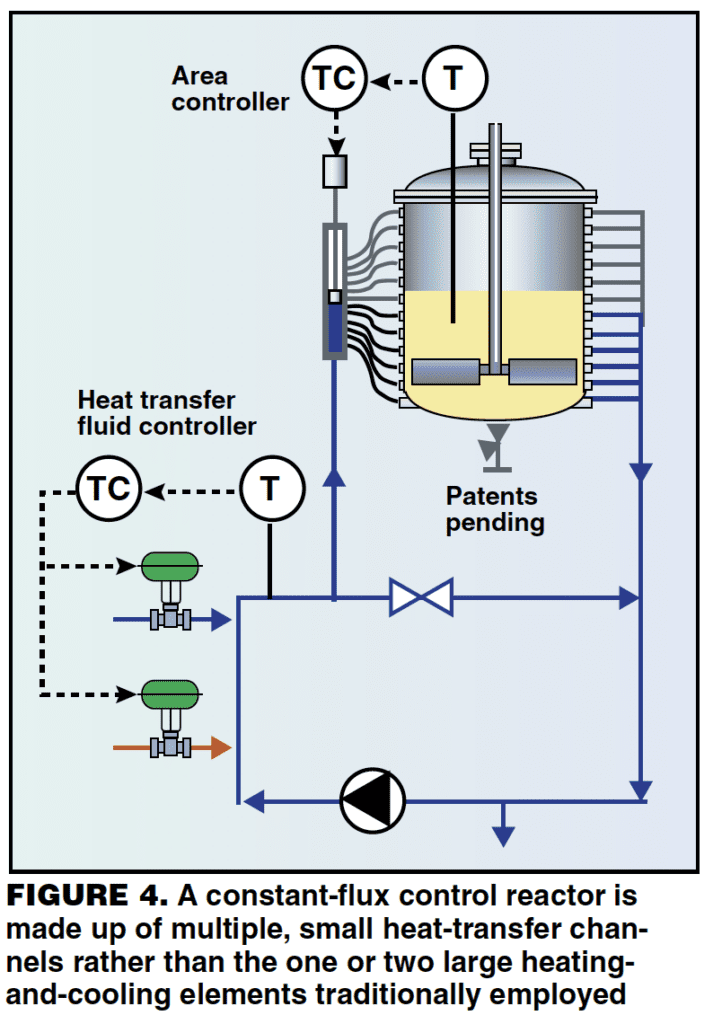
The constant flux jacket. This configuration is made up of multiple, small heat-transfer channels rather than one or two large heating and cooling elements. Each heat transfer channel is a pipe, which is clamped or welded around the external surface of the vessel (or in some cases these may be internal coils) and is connected to a multi-port piston valve. A controller regulates the valve position and as it does so, heat transfer elements open and close in a cascade fashion. A second control loop regulates the temperature of the heat transfer fluid. By varying the number of coils in service, the control system is effectively regulating heat transfer area. This means that it can alter heating or cooling power without changing the jacket heat flux (hence constant flux).
The constant flux jacket has significant advantages over conventional jackets. Transient hot or cold spots associated with heat load changes can be eliminated, since the heating or cooling power can be altered without changing the jacket temperature. The jacket also functions like a split jacket, which can be set at any height to suit the process level. This can even vary as the process level changes.
The turnover rate of the heat transfer fluid in the constant flux jacket is very high. A 5,000-L constant flux reactor, for example, has a jacket turnover rate that is equivalent to, or better than, a conventional 1-L laboratory reactor. This means that temperature-control dynamics are virtually unaffected by scale.
The control characteristics of the constant flux jacket have been compared with conventional reactors in both simulation studies and live tests. In both cases, the control capabilities of the constant flux jacket have been found to be significantly faster and more stable than conventional vessels. Figure 5 profiles a reaction between butanol and acetic anhydride using 1,1,3,3-tetramethylguanidine as the catalyst. The experiment was repeated in a conventional 5-L reactor and a 10-L reactor with a constant flux jacket.
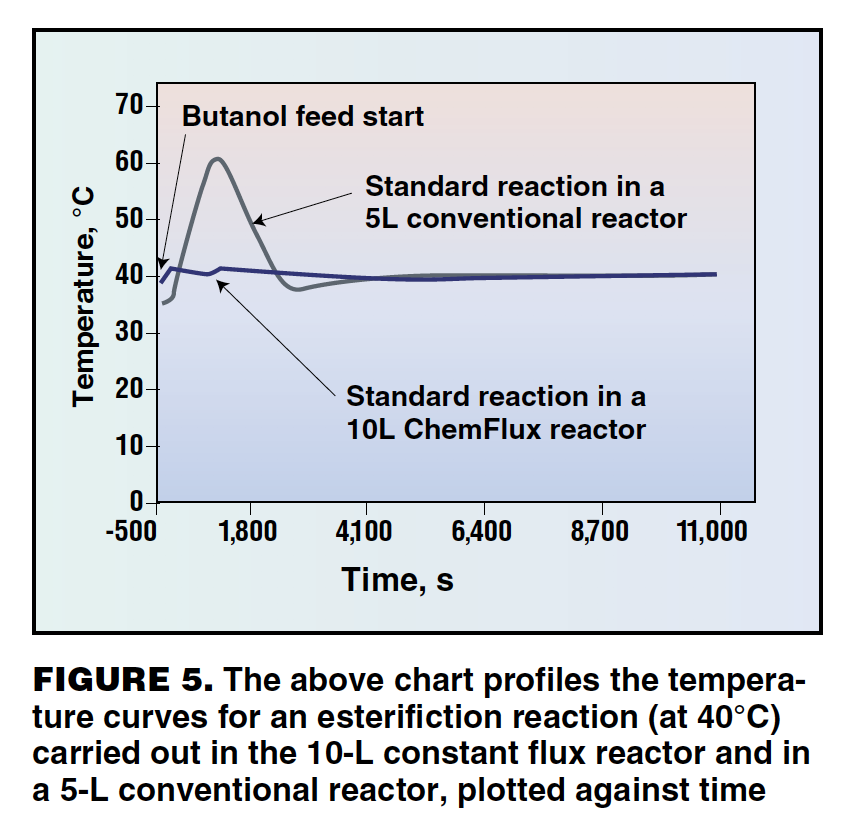
When the reaction was performed in a conventional 5-L reactor (grey line) the initial temperature dip is caused by the cooling effect of the butanol addition (which is added at room temperature). As the reaction starts, the process temperature suffers a significant overshoot followed by a small but prolonged undershoot. The blue line shows the same experiment performed in a 10-L constant flux controlled reactor. Even though this vessel is larger, the overshoot and undershoot effects are virtually eliminated to give near perfect temperature control.
Process monitoring
The function of reactor jackets is to add or remove heat. Where this heat can be measured, however, it can also serve as a valuable PAT tool. Unfortunately, erratic temperature shifts associated with temperature control in conventional reactor jackets make such measurements unreliable and difficult to perform. Constant flux jackets, by contrast, have essentially constant jacket temperatures, and thus, the heat can be measured by a simple heat-balance technique.
Figure 6 shows the results of a heat balance measurement performed during a chemical reaction. In this experiment, the change in enthalpy was monitored during the formation of butyl acetate. The reaction was repeated at three different temperatures and the enthalpy trends show good correlation with changes in the concentration of butyl acetate determined by gas chromatography.
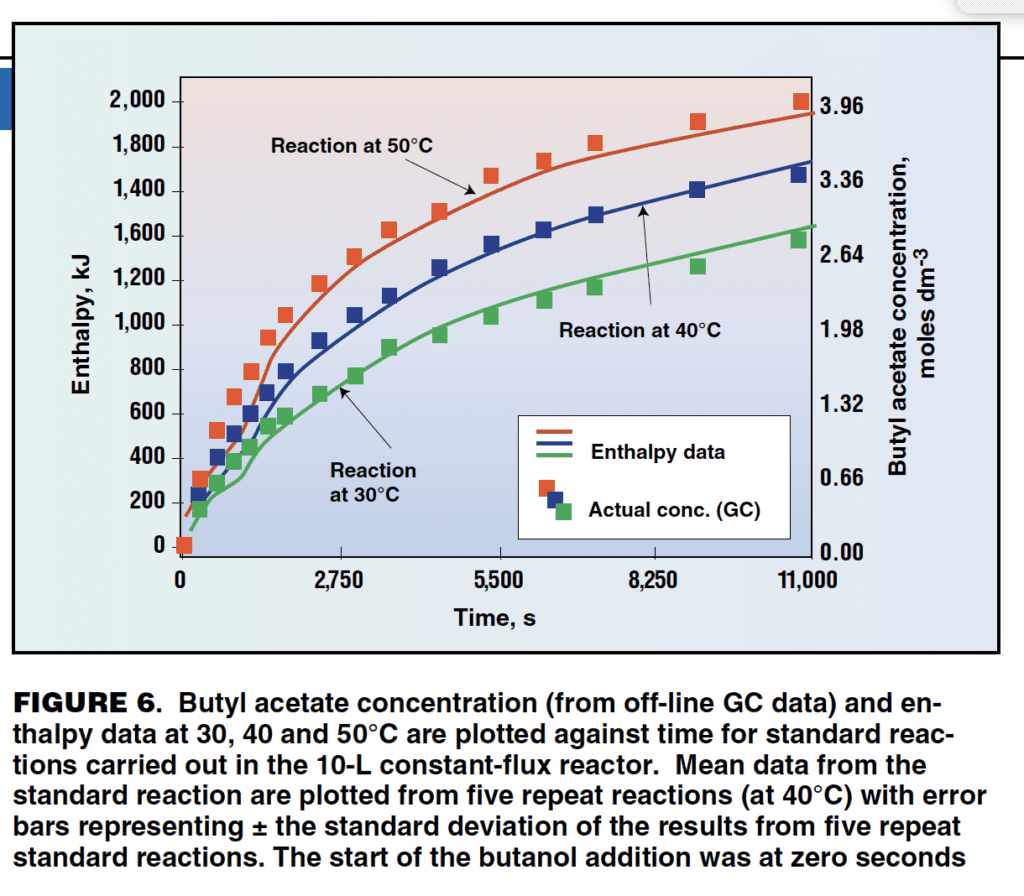
Heat balance measurement is an effective way of sensing change in batch reactors. As a PAT tool, it is multi purpose, simple to use, non-intrusive and requires very little calibration. Heat balance measurement can be used for monitoring process change, internal wall temperatures and heat transfer coefficients. The same set of instruments and calibration settings are used whether monitoring chemical reactions, crystallization or bioprocesses. The practical uses for heat balance measurement include end-point detection, addition control, crystallization control and control of bioreactors. In heating or cooling duties (for example, evaporation, distillation, heat up and cool down) the wall temperatures in the vessel can be monitored from the temperature and heat balance data. This allows the user to regulate the jacket temperature according to the observed internal-wall temperature. This means faster heating and cooling without exposing the product to damage from excessively hot or cold wall temperatures.
Conclusions
Constant flux control affects the mechanical design of the external cooling jacket and temperature control valve. With this design, temperature control is not only faster and more stable, but the problem of hot and cold spots in the jacket is also eliminated. The stability of the jacket temperature makes accurate and sensitive heat-balance measurement possible. This provides a non-intrusive tool for monitoring most of the common unit operations carried out in batch reactors. Furthermore, as the constant flux technology is independent of reactor size, the benefits can be realized at development, pilot and manufacturing scales.
Edited by Matthew Phelan
Acknowledgements
The authors acknowledge the contributions from Katy Basford, Martin de Cecco and Maryann Ehly with respect to some information used in Figure 6.
AuthorsRobert Ashe (Ashe Morris Ltd., The Heath Business & Technical Park, Runcor, Cheshire, WA7 4QX, U.K.; Phone: + 44 (0) 1928 51 54 54; Email: [email protected]) is a chemical engineer and has worked in the batch process industries for 30 years. For most of this time he has been closely involved with the design and operation of batch process equipment. In 1990 he started working on the idea of variable-area heat transfer surfaces for batch vessels. In 2000 he co-founded a company with David Morris and together they built and tested a variable area batch reactor. Variable area reactors have now been successfully developed for use in lab, pilot scale and manufacturing systems. He is continuing to develop uses for this technology as a process analytical tool. David Littlejohn (Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, Scotland, G1 1XL, U.K.; Phone: +44 (0)141 548 2067 Fax: +44 (0)141 548 4212; Email: [email protected]) received his B.S. and Ph.D. in chemistry from the University of Strathclyde. After a period working at ICI, he returned to Strathclyde in 1981 and has been Professor of Analytical Chemistry at the University since 1988. He is currently head of the Department of Pure and Applied Chemistry. His activities in the development and application of inline, online and non-invasive methods of process monitoring cover a range of techniques and data analysis methods, including NMR, NIR, MIR, and Raman spectrometries, acoustic techniques and mass spectrometry. David is a founding member of the Centre for Process Analytics and Control Technology (CPACT), a multi-disciplinary industry-university collaboration devoted to research, technology translation and training in realtime monitoring, process optimization and control. Alison Nordon (Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, Scotland, G1 1XL, U.K.; Phone: +44 (0)141 548 3044; Fax: +44 (0)141 548 4212; Email: [email protected]) is a lecturer and Royal Society University Research Fellow in the Department of Pure and Applied Chemistry at the University of Strathclyde. Alison obtained a B.S. (Hons) in Chemistry and a Ph.D. in solid-state NMR spectroscopy from the University of Durham. She then moved to the Department of Pure and Applied Chemistry at the University of Strathclyde where she held research fellow and senior research fellow posts with the CPACT. In 2004, she was awarded a Royal Society University Research Fellowship to work on the development of non-invasive active acoustic techniques for process monitoring and control. Alison’s current research interests are the development of non-invasive and in situ spectroscopic measurements (NMR, optical techniques and acoustics) for process monitoring and control, chemometrics and signal processing. Pamela Allan (Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, Scotland, G1 1XL, U.K.; Email: [email protected]) received her undergraduate M.S. in ‘Forensic and Analytical Chemistry’ at the University of Strathclyde in Glasgow. She has held a studentship from CPACT to carry out research with Professor Littlejohn at Strathclyde since October 2004, and is now finalizing her Ph.D. thesis. Allan is an affiliate member of the Royal Society of Chemistry and a member of the Society of Chemical Industry. |