Biotechnology plays a key role in sustainably feeding a growing global population
How are we going to feed 10 billion people using 35% less land by 2050? This was the question posed by Ian Roberts, chief technology officer at Bühler AG (Uzwil, Switzerland; www.buhlergroup.com) at a (virtual) press event last December. Reducing agricultural land usage is necessary to restore forests and wetlands, not only to sequester CO2, but also to ensure the biodiversity in our ecosystem that is critical to life, explained Roberts. “This is where we believe new protein sources come into play, and we have to focus on them deeply,” he said.
Bühler is not alone in this focus. In fact, the number of new startup companies developing new and alternative protein sources is growing explosively, not to mention the growing participation — and investment — by traditional food, pharmaceutical and chemical companies.
New protein sources
There are several fronts attempting to meet these challenges: traditional plant-based proteins (vegetarian and vegan meat-like products); cultured meat (growing real beef, pork, chicken, seafood and more from cells); and alternative protein sources (derived from insects or produced by genetically modified organisms and plants). There is also significant development work in controlled-environment agriculture and aquaculture (fish farming). What unifies each of these areas is feeding the population more sustainably, says David Ziskind, director of Engineering at Black & Veatch NextGen Ag (B&V; Overland Park, Kan.; www.bv.com).
“Sustainability has always been a focus at Black & Veatch, which has over 100 years of experience in infrastructure projects in wastewater treatment, oil and gas and more,” Ziskind says. About five years ago, the company established the NextGen Ag group to apply its know-how of process and technology scaleup to these emerging food technologies. “The intersection of our activities with these new projects is perfect,” he says. “After all, when you talk about scaleup for these new food products, there is an infrastructure component to it. You need a certain level of infrastructure to support it, from a water, wastewater, electricity standpoint. And there is definitely a sustainability angle to it. With traditional food and beverages, sustainability has been something like a bolt-on added at the top. In this new space, we see new companies — many with dedicated sustainability officers — essentially reinventing our food supply, and sustainability is being thought of from the bottom up,” says Ziskind.
Cultured meats
Although there is a tremendous amount of research and development (R&D) going on in new protein sources, this article focuses on cultivated meat, a topic that has captured the attention of the general public, as well as investors. According to the latest data, released last month by the Good Food Institute (GFI; Washington, D.C.; www.gfi.org), a record $5 billion was invested in alternative proteins in 2021, a 60% increase over 2020. Cultivated meat and seafood companies secured $1.4 billion in investments in 2021 — three times more than the $400 million raised in 2020. There are now more than 70 startup companies focused exclusively on developing cultivated meat inputs or end products, according to GFA’s “2020 State of the Industry Report: Cultivated Meat,” which was published last year (the 2021 report is expected to be published this month).
Some of the new cultured-meat companies were formed and are headed by biotechnologists and former cardiologists, because the technology used has roots in stem-cell research and treating heart patients.
Cultured meat is real meat that is grown from animal cells. Unlike the meat, fish, chicken and pork found in grocery shops, no animals need to be slaughtered to produce cultured meat. Instead, cells taken from living animals are grown in bioreactors or cultivators (Figure 1) at high densities and volumes. The cells are fed an oxygen-rich cell culture medium made up of nutrients, such as amino acids, glucose, vitamins, and inorganic salts, and supplemented with proteins and other growth factors, according to GFI’s website.

FIGURE 1. Cultured meats are produced in bioreactors (cultivators), such as the one shown here
Changes in the medium composition, often in tandem with cues from a scaffolding structure, trigger immature cells to differentiate into the skeletal muscle, fat and connective tissues that make up meat. The differentiated cells are then harvested, prepared and packaged into final products. This process is expected to take between two and eight weeks, depending on what kind of meat is being cultivated, according to GFI.
Although cultured meat and seafood has not yet been approved for human consumption by regulators around the world — with the exception of Singapore — there have been countless tasting events in California, Israel and elsewhere, and some restaurants offer dishes on their menu for customers willing to sign waivers regarding unknown health issues associated with such products.
While there are many uncertainties related to government approvals, labeling of future products that contain cultured meat, and scale up of production, there are many arguments in favor of this new source of meat. Cultured meat is sometimes referred to as “clean meat,” because it is produced under sterile conditions and is free of hormones and other chemicals that can be found in some products from traditional livestock farming or seafood from polluted lakes or oceans, not to mention the risk of pandemics, antibody resistance and food insecurity. From a moral perspective, no animals need to be sacrificed. Perhaps the best argument in favor of cultured meat is the sustainability aspect referred to earlier.
The first lifecycle analysis that uses primary data from multiple cultured-meat companies and associated companies along the supply chain was published in February 2021 by CE Delft (the Netherlands; www.cedelft.eu). The study found that significant reductions in CO2 equivalent (CO2 eq) emissions (by up to 92%), land usage (by up to 95%) and water usage (by 78%) can be achieved for all types of cultured meats — some more than others — when compared to conventional farming methods. So even though the environmental benefits of plant-based meats are greatest (99% reduction in CO2 eq emissions for plant-based beef compared to conventional beef from beef cattle), “not everyone needs to become vegan” to contribute to a sustainable diet, says Bühler’s Roberts.
First pilot plants operating
Although the cultured meat industry is still very young, the first mini and pilot-scale production plants are now starting up and operating, with further scaleup anticipated soon (Figure 2). The following is a brief overview of some of the activity within the past six to eight months.
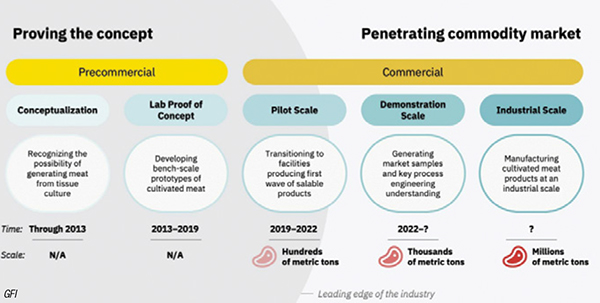
FIGURE 2. This diagram shows the projected timeline for cultured food products
Last June, Future Meat Technologies (Rehovot, Israel; www.future-meat.com) opened its first pilot plant (Figure 3), which has a capacity to produce cultured chicken, pork and lamb products, all produced without the use of animal serum or genetic modification. The company says its process enables fast production cycles that are about 20 times faster than traditional animal agriculture, while using 99% less land and 96% less water, as well as generating 80% less greenhouse-gas emissions. The company is also working on the production of beef, and plans to bring products into the market this year, as well as “scouting several locations in the U.S. for its projected large-scale production facility,” the company says. In December 2021, Future Meat raised $347 million in Series B round financing, which was co-led by ADM Ventures, the investment arm of the Archer-Daniels-Midlands Co. (ADM; Chicago, Ill.; www.adm.com).

FIGURE 3. An inside view of the the first industrial cultured meat facility in Rehovot, Israel
According to Future Meat, its proprietary technology is based on stainless-steel fermenters that continuously remove waste products generated by “immortal” tissue cells, thereby maintaining a constant physiological environment that supports rapid, natural, proliferation of animal cells. This connective-tissue method has been shown to be more robust and efficient than others using stem cells, and its rejuvenating fermenters can recycle over 70% of the nutrients, the company says.
Also last June, Wildtype Foods (San Francisco, Calif.; www.wildtypefoods.com) started operations at its first pilot plant in San Francisco, Calif. for the production of salmon products. The facility also features a sushi bar (tasting room) for visitors.
Meanwhile, Upside Foods (Berkeley, Calif.; www.upsidefoods.com) completed construction of its Engineering, Production and Innovation Center (EPIC) in Emeryville, Calif. last November. The 53,000-ft2 campus is designed to produce any species of meat, poultry and seafood directly from animal cells. Patented, custom-made cultivators (Figures 1 and 4) can produce over 50,000 lb/yr of finished product, with a future capacity to over 400,000 lb/yr. Initially, the company started producing meat for its group of restaurant partners. The company — formerly known as Memphis Meats — introduced the world’s first cultivated meatball in February 2016 and the world’s first cultivated poultry in March 2017.

FIGURE 4. A view from the control room of Upside Foods’ Engineering, Production and Innovation Center in Emeryville, Calif.
Also in November, Shiok Meats (Singapore; www.shiokmeats.com) officially opened its first-of-a-kind R&D facility for cultivated seafood. B&V collaborated with Shiok Meats for the conceptual design and layout of this facility, which will help Shiok Meats, the world’s first cultivated crustacean company, scale-up production of cell-based crustacean meat products, targeting commercialization by 2023.
“Building the Mini Plant is a big milestone for us,” said Sandhya Sriram, group CEO & co-founder, Shiok Meats at the opening of the plant. “Our production facility, which is due in the next 18 months, will be an extension of this Mini Plant in terms of engineering design and foundation. This new facility allows us to scale the cultivated seafood production gradually and strategically to ensure a comprehensive manufacturing model and top-notch products,” she said.
Meanwhile BlueNalu, Inc. (San Diego, Calif; www.bluenalu.com) is scaling up to pilot scale. “The completion of our pilot production facility and the preparation to achieve regulatory requirements needed by the U.S. FDA for our cell-cultured seafood products are both currently in process,” says Lou Cooperhouse, the company’s co-founder, president and CEO. “Our new BlueNalu Innovation Center will be 40,000 ft2 and will incorporate a processing environment that enables production of limited volumes under Good Manufacturing Practices (GMP) conditions and global best practices in food safety. This will enable us to continually ideate, innovate and introduce new species, and new forms and packaging options for our customers and consumers,” he says.
“We have developed a multi-species platform technology in which we are initially developing finfish products that include pacific bluefin tuna,” explains Cooperhouse. “We are specifically targeting species that are overfished, primarily imported and difficult to farm-raise. It is our goal, as a result of this strategy, to reduce fishery pressure, complement the existing seafood supply chain, displace the need for imports, create jobs, enhance food security, and offer the ultimate seafood experience in each region we go to market,” he says.
Meanwhile, The Cultured Food Innovation Hub will start up in Kemptthal near Zurich this year. This new facility for developing cellular agricultural products was established by Swiss companies Givaudan (Vernier; www.givaudan.com), Bühler and Migros (Migros-Industrie Migros-Genossenschafts-Bund; Zurich; www.mgb.ch). The Cultured Food Innovation Hub aims to accelerate the development and market penetration of cellular agriculture products.
The new entity provides facilities and knowledge to accelerate other companies on their cultured meat, cultured fish and seafood, and precision-fermentation developments. The Hub will be equipped with a product-development laboratory, as well as cell culture and bio-fermentation capabilities to help start-ups develop and go to market with the right product.
Media costs
Growth media is estimated to account for 50–90% of production costs of cultured meat, so efforts are underway to reduce these costs, which will ultimately make cultivated meat products more affordable. Last October, for example, Mosa Meat (Maastricht; www.mosameat.com) and Nutreco N.V. (Amersfoort, both the Netherlands; www.nutreco.com) were awarded nearly €2-million funding from the European REACT-EU recovery assistance program to advance cellular agriculture and bring cultivated beef to the E.U. market. The funding for this “Feed for Meat” project is being used to address the “basal” or base media in which beef cells grow. By moving away from pharmaceutical-grade products and instead using feed- and food-grade byproducts from Nutreco’s supply chain, Mosa Meat believes it can substantially lower costs of basal media. Mosa Meat introduced the world’s first cultivated beef burger in 2013, and has since received backing from Blue Horizon, Bell Foods Group, Nutreco, Mitsubishi Corp., Leonardo DiCaprio, and others.
In a related project, Meatable (Delft; www.meatable.com) entered a joint-development agreement last September with Royal DSM N.V. (Heerlen, both the Netherlands; www.dsm.com) to develop growth media for cultivated meat.
Last December, Wacker Chemie AG (Munich, Germany; www.wacker.com) entered a partnership with Aleph Farms (Rehovot, Israel; www.aleph-farms.com) to develop streamlined production processes for growth medium proteins, which “we believe are key to bringing down the overall cost of cultivated products,” says Oliver Minge, head of Innovation Biosolutions at Wacker. “We also believe that as the industry grows, we will need to build up a supply chain for these compounds that satisfies the food industry’s requirements and regulations.”
Scaleup challenges
Supply chain issues may also hinder progress as companies complete their pilot phase and look to build commercial plants. Scaleup will require good planning in advance, says B&V’s Ziskind. One of the challenges will be the capacity to manufacture more bioreactors. “There is a certain lead time for a large-scale piece of equipment, and it might take a year or even longer before you get it,” explains Ziskind. “Think of all the innovation and the movement in this space. You could order a piece of equipment today to be delivered in 2023, but the way things change, it might not be the right size or isn’t the right technology by the time it arrives. Companies need to be forward-focused,” he says.
Nevertheless, “every basic technology needed for cultivated meat production has been developed and there is no missing part or aspect that would need to be invented first [before scaling up to industrial scale],” says Wacker Chemie’s Minge. The main technical challenge he sees today is the following: “Unlike microbial cells, mammalian cells take longer to grow and are generally more prone to impurities. They therefore need a higher level of cleanness when being grown. Maintaining a clean environment, however, becomes more difficult and more expensive, the larger your overall production volumes are,” he says. Minge believes the largest vessels for mammalian cell cultures in the pharma business today are in the range of 20,000 L. “Some people argue that volumes above 100,000 L would be hard to achieve. I am more optimistic here: on the one hand, it has been shown that for another very delicate type of cell (plant cells), production on a 100,000-L scale is feasible; on the other hand, I think that there are also alternatives to scaling up a batch process, for example scaling out or continuous production.”