This column is based on “Economics of Aniline Production from Nitrobenzene,” a report published by Intratec. It can be found at: www.intratec.us/products/aniline-production-processes.
Aniline, also known as amino benzene or benzenamine, is an aromatic amine with the formula C6H5NH2. It is mainly used as a raw material in the production of methylene diphenyl diisocyanate (MDI), an intermediate in polyurethane manufacture. Aniline is also used as an intermediate for dyes and pigments, explosives, agricultural chemicals and pharmaceuticals.
The process
The following paragraphs describe aniline production from nitrobenzene via a liquid-phase hydrogenation process, similar to the one owned by DuPont (Wilmington, Del.; www.dupont.com). The process can be divided into three main parts: nitrobenzene hydrogenation, dehydration and purification. Figure 1 presents a simplified flow diagram of the process showing the main pieces of equipment.
Nitrobenzene hydrogenation. Nitrobenzene (mononitrobenzene or MNB) is fed with hydrogen into a plug-flow tubular reactor containing a noble metal catalyst supported on carbon. The hydrogenation is carried out in the liquid phase and the nitrobenzene conversion to aniline is near 100% in a single pass.
Dehydration. The reactor effluent is virtually free of nitrobenzene due to the high conversion of the reaction. The hydrogen excess is separated from the reactor effluent and the liquid product is directed to a dehydration column. In this column, the water generated is removed as the overhead product and the bottoms stream is sent to the purification area.
Purification. In the purification area, heavy impurities (tars) are separated from the crude aniline stream by the bottom of a distillation step. The final product obtained as the distillate of the column is high-quality aniline, with purity above 99.95 wt.% and containing less than 0.1 parts per million (ppm) of nitrobenzene by weight.
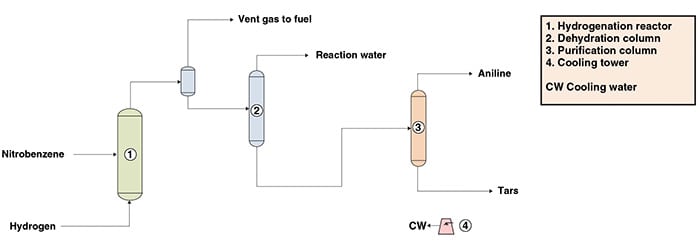
Figure 1. The diagram shows aniline production from nitrobenzene via a liquid-phase hydrogenation process
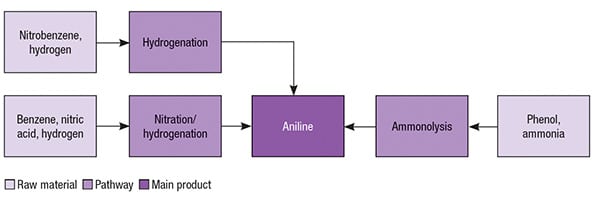
Aniline pathways
Aniline was first commercially produced using nitrobenzene as starting material in 1930s. This pathway remains the most common for aniline production today. Currently, almost all existing plants producing aniline from nitrobenzene are integrated with facilities to produce nitrobenzene from benzene. The other existing production pathway for aniline is based on phenol as the starting raw material. Figure 2 illustrates such aniline production pathways.
Economic performance
The total fixed capital estimated to construct a plant to produce 350,000 metric ton/yr of aniline in the first quarter of 2015 in the U.S. is about $200 million. The total fixed capital estimated includes the inside and outside battery limits (production units, storage installations, utilities facilities and auxiliary buildings).
Edited by Scott Jenkins
Editor’s Note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.