Quaternary diaphragm pumps deliver the low pulsation and shear that is critical to these biopharmaceutical and continuous-manufacturing operations
Biopharmaceutical, pharmaceutical and nutraceutical manufacturing may come in a wide variety of forms, but every iteration of unit operation must adhere to an unbending set of operational parameters and structures if the desired outcome — a viable, contaminant-free drug suitable for human or animal administration — is to be realized. It is all about “controlled fluid transfer.”
Three of the more common unit operations within the biopharmaceutical-manufacturing universe are chromatography, virus filtration and tangential flow filtration (TFF). In order for these unique operations to be implemented successfully, though, the operator must be aware of their specific operating characteristics. For example, chromatography requires constant fluid flowrates during their operations, but may have varying pumping pressures. Virus filtration, on the other hand, will feature constant pumping pressures, but flowrates will change as the filters become clogged or fouled. And in TFF, the main challenge is attempting to keep the flowrate and pressure unchanging throughout the process. All these characteristics are also fundamental for inline blending, a process commonly used not only in the biopharmaceutical segment, but also in the chemical, pharmaceutical and food and beverage industries, where constant flow and pressure ensure the quality of the mixture.
While fluid transfer is taking place in any of these specific unit operations, it is important to know that the materials that are being transferred can be highly sensitive and delicate (and, in many cases, expensive), meaning that the pumping action must be low-pulsating and low-shear, lest the material be damaged.
This article examines the material-handling challenges pertaining to flowrate and pressure in chromatography, virus filtration, TFF and inline blending processes, and illustrates why the design and operation of the quaternary diaphragm pump — rather than other technologies, such as the lobe or peristaltic (hose) pump — make it suitable for use in critical biopharmaceutical-manufacturing applications. Additionally, the article shows how the quaternary diaphragm pump’s ability to operate consistently whether in a fixed stainless-steel production regime or in the increasingly popular single-use applications gives it the versatility to optimize biopharmaceutical-manufacturing maintenance, downtime, changeover and operational costs.

Figure 1. Shown here is a chromatography application utilizing three quaternary diaphragm pumps (center, right)
The unit operations
Chromatography columns. A typical chromatography column, whether it is glass, steel or plastic, is filled with resins that are compressed in a certain format through which the product-containing feedstream flows and purifies the product by selective adsorption to a stationary phase (resin). Chromatography columns contain complex target-product adsorbing media that need careful handling. Protein A resin, for example, can cost as much as $10,000/L, making proper feeding of the resin extremely important.
Quite often, more than one buffer is required, which creates the need to use two or more pumps. In this application, high- and low-salt buffers are mixed continuously and with changing ratios in order to affect the adsorption of the target molecule to the chromatography resin. Because of this, precise pumping is required to achieve the right pH/conductivity conditions for specific adsorption and high-resolution purification. For example, a Buffer A and a Buffer B can be used to create a gradient that ranges from a low-salt buffer to a high-salt buffer in a linear fashion. Specifically, the process will begin with Buffer A producing 95% of the flow and Buffer B the remaining 5%. Over the course of the operation, the flowrates of Buffer A and Buffer B will decrease and increase in a linear fashion (90% for A and 10% for B, 75% for A and 25% for B, all the way to 5% for Buffer A and 95% for Buffer B) [1].
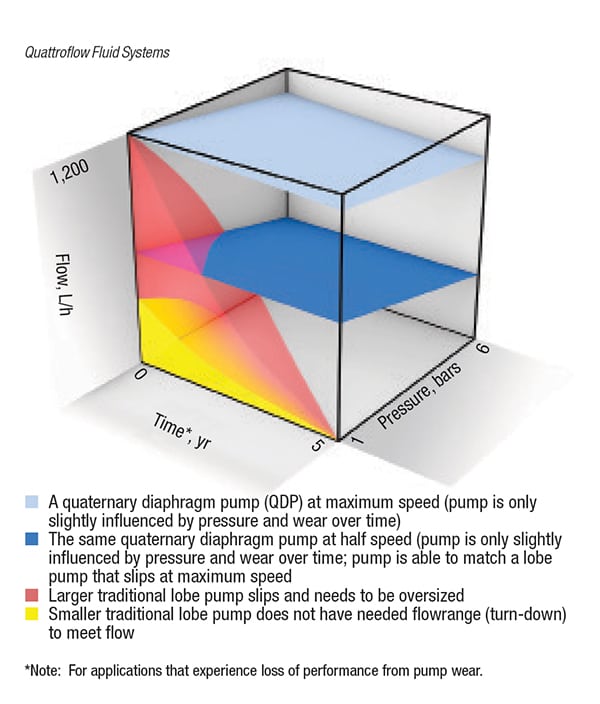
This requires a pumping technology that can produce a highly accurate flow with a high turndown ratio that can deliver low and high flowrates as the elution stage continues, and ensure constant flow. Pump pulsation should also be minimized to prevent disturbance of the packed column [2]. If the pump is not able to meet these requirements, the correct buffer concentration may not be attained. Also, if the pumping action produces excessive pulsation, the buffers can be susceptible to experiencing spikes in their conductivity.
This can affect the purification level of the product, as the salt level in the buffer could be compromised. Also, during the loading of the sample, it is not uncommon for the system’s backpressure to increase. Pumps that do not slip offer benefits in these situations since their flowrates will remain consistent and the linear velocity will remain stable. Simply put, a pump with minimal slip will have a more easily controlled flowrate that will need only incremental adjustments to the pump’s speed.
Virus filtration.In biopharmaceutical manufacturing, virus-filtration systems are used to ensure the viability and safety of the drugs that are produced through the removal of potential contaminants from products that are created using cell cultures. Whereas chromatography features constant flowrates and variable pressures, the operation of virus-filtration systems is the opposite — most virus-filter applications use constant pressures with variable flows. In other words, you may have to raise and lower the operation’s flowrate or speed in order to maintain a constant pressure.
As mentioned, the flows change as the virus filter becomes clogged. Most typical virus-filtration systems run at a constant pressure, for example, 2 bars (29 psi), due to the nature of the tight pores in the filtering medium, but the flowrates will decrease as the filter’s pores become fouled. When this happens, the flowrate will not decrease in a linear fashion, which will adversely affect the performance of the filter, product yield and overall quality.
Some virus filters have been designed with a flux-decay capacity of up to 90% of the starting fluxrate, which requires a pump that has both a high turndown ratio and produces minimal pulsation in the pumped fluid. Evaluation of viral clearance strategies requires demonstration of the equivalence of scalability from bench to manufacturing scale and vice versa [ 3]. Spiking studies for virus-filtration use a pressure vessel with a small surface area, which can be as little as 5 cm 2 and demand a pump that has low-shear and low-pulsation operation if commercial-scale production levels are to replicate the small-scale studies. The use of low-pulsing pumps in these circumstances can ensure that pressure conditions during validation of the particular filter are not outside of the validated range.
Tangential flow filtration (TFF). Also known as cross-flow filtration, in TFF the biologic feedstream flows tangentially across the filter membrane at positive pressure. As it passes across the membrane, the portion of the feedstream that is smaller than the membrane’s pore size passes through the membrane. This is different from what is known as normal-flow (NFF), or “dead-end,” filtration, in which the feed flows entirely through the filter membrane, with the size of the pores determining which portion of the feed is allowed to pass through and which will remain trapped in the filter membrane.
TFF is different from NFF in biologic applications because the tangential motion of the fluid across the membrane prevents molecules from building up a compact gel layer on the surface of the membrane. This mode of operation means that a TFF process can operate continuously with relatively high protein concentrations with less fouling or binding of the filter.
To scale up a TFF process, there are two variables that need to be successfully controlled. Recirculation (cross-flow) is required to minimize formation of the gel layer and pressure as the driving force to push the permeate through the membrane. The recirculation rate needs to work in conjunction with the pressure (known as the trans-membrane pressure, or TMP, which is the average amount of pressure that is applied to the membrane). Maintaining a constant TMP is critical because if it is too high, it can cause gel-layer formation that cannot be removed by recirculation, and if it is too low, it results in low flux that will reduce process efficiency (for more information on crossflow filtration, see “Crossflow Membrane Filtration Essentials,” Chem. Eng., April 2017, pp. 49–59; www.chemengonline.com/crossflow-membrane-filtration-essentials).
Inline blending. Also known as continuous blending or inline mixing, inline blending systems, as well as inline buffer dilution, correspond to a new standard for just-in-time-production and reflect the next step in the evolution of continuous-production technology. In this type of process, liquid ingredients are fed proportionally to one main stream and are instantly mixed, since they are being transferred within a common manifold.
In order to obtain exactly the right product, this process requires good metering and volumetric efficiency capabilities that can facilitate the automation of the system. This is different from batch blending, where the product in the tank can be adjusted after measurement in the laboratory according to the parameters defined in the recipe. When this production is continuous, the blending is instantaneous and there is almost no space or time available for corrections.
In this instance, pumps that deliver low-pulsation flow characteristics will perform most reliably by decreasing the fluctuation in the variables. So, in considering the functional design of chromatography columns, virus-filtration systems, TFF systems and inline blenders, the common thread in guaranteeing efficient, reliable, cost-effective operation is found in identifying and using a pump technology that is capable of producing both low-pulsation and low-shear operation despite varying flowrates and pumping pressures that are accompanied by high volumetric efficiency.
Determining Flow and Pressure Pulsation of Quaternary Diaphragm Pumps
Using water at ambient temperature as the medium, pressure and flowrates were recorded for a quaternary diaphragm pump at free flow, 2 bars, 4 bars and 6 bars (29, 58 and 87 psi) pressure and at motor speeds of 250, 1,000 and 2,000 rpm. The measuring frequency was one measuring point per second (1 Hz), and the duration measuring point was approximately five minutes. Figure 2 shows the results that were observed.
The maximum flow pulsation measured by the quaternary diaphragm pump was 13 L/h (3.4 gal/h), which was approximately 1.2% of the average flowrate. Regarding pressure pulsation, the maximum value was 0.17 bar (2.5 psi), which is 4.2% of the average pressure. These results indicate that operation of quaternary diaphragm pumps is quite capable of minimizing harmful pulsation in critical biopharmaceutical-manufacturing and handling applications.
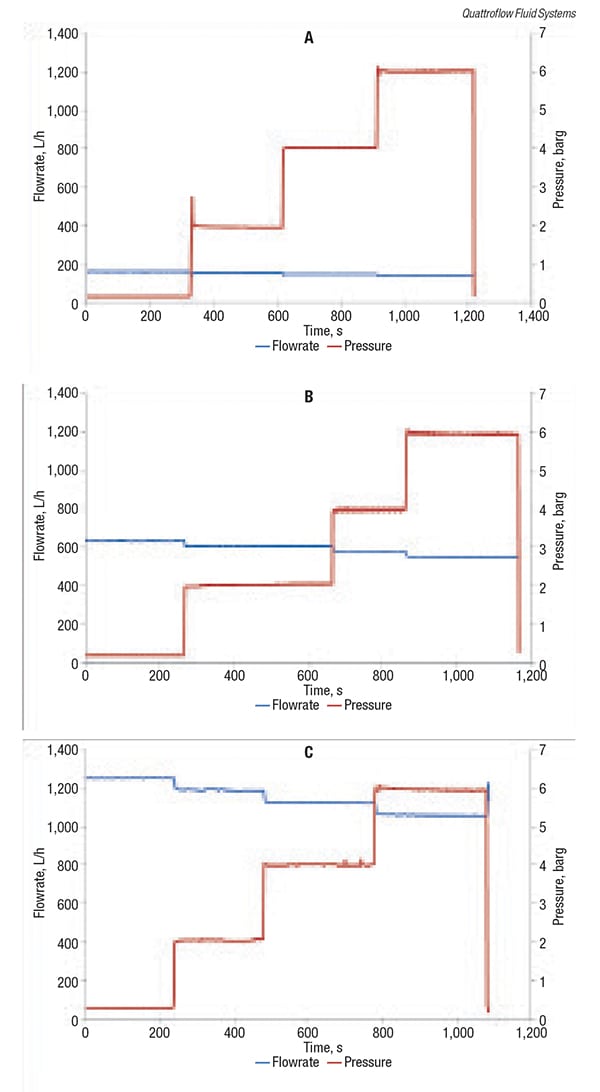
Figure 2. These graphs show the flow behavior of a quaternary diaphragm pump as at different pressures for three different pump speeds: 250 rpm (a), 1,000 rpm (b) and 2,000 rpm (c) (see sidebar text for details)
The challenge
With these operational requirements in mind, over the years, various pump technologies have been tested and used for chromatography, virus filtration, TFF processes and inline blending. Two that are among the more popular choices when positive displacement is required are lobe and peristaltic pumps. Both, however, have been found to feature operational inefficiencies that may make them insufficient for use in the processes described earlier.
Lobe pumps. Since many biopharmaceutical materials are contained in a low-viscosity aqueous solution, lobe pumps may not be a good choice because slippage can occur during their operation. Slippage can vary between 10% to 100%, depending on the system’s backpressure. Slip will also result in increased shear damage and energy consumption, and if used in a long-duration recirculation loop, such as a TFF filtration system, there can be noticeable heat addition to the product, which can require significant cooling efforts to protect the product from overheating.
Lobe pumps also have mechanical seals, which can result in a controlled product leak and do not provide full containment unless special (and oftentimes expensive) seals and seal barriers are used. The sterility required in biopharmaceutical handling also means that no outside contaminants can be introduced into the purification process, which is something that pumps with mechanical seals cannot reliably ensure.
Additionally, the necessary contact between a lobe pump’s internal parts can lead to wear and the generation of particles that can result in product contamination. Solid particulate matter, such as undissolved salt crystals, can cause severe damage to the lobes, resulting in damage to the entire manufacturing batch. Lobe pumps may ultimately cost more to operate because of the increase in power required to overcome the pump’s slippage.
Peristaltic (hose) pumps. The main shortcoming of peristaltic pumps is also the most obvious: their method of operation will undoubtedly produce pulsation, and, as noted, pulsation is undesirable in biopharmaceutical manufacturing. Peristaltic pumps also have limited flow and pressure-handling abilities. For example, they cannot reliably produce the higher discharge pressures (such as 4 bars, or 58 psi) that are required in some fluid-handling applications.
They are also known to release some small quantity of hose material — in a process known as “spalling” — into the pumped product, which can compromise its purity. If the spalled hose material makes its way to the filter, it can foul the filter, making its operation less efficient than it need to be, and will also lead to contamination. Also, inconsistency of flowrate will result due to mechanical deformation of the hose during the pumping process. In the end, the shortcomings of lobe and peristaltic pumps come down to two main factors:
- If there is shear, which is common in lobe pumps, you will damage the pumped material
- If there is pulsation, an operational certainty with peristaltic pumps, you won’t have even flow, and without even flow, you won’t have accurate flow
A solution
The quaternary diaphragm pump’s method of operation allows it to gently, safely and securely convey low-viscosity aqueous solutions and biopharmaceutical materials that are highly sensitive to shear forces and pulsation while being pumped. Since the four-piston design of the pump does not require any mechanical seals or wetted rotating parts, total product containment is ensured without any abrasion or generation of particulate matter. The pump’s method of operation also produces risk-free dry-running and self-priming capabilities with high turndown ratios. A pump technology with high turndown ratios allows for the creation of a broad flow range, which makes the pump applicable for utilization in a wide range of process applications.
With regard to specific unit operations, quaternary diaphragm pumps can be used to pack chromatography columns and then pump the biopharmaceutical material through the column, both of which are critical concerns that require low pulsation with accurate and constant flowrates and pressures. In TFF applications, quaternary diaphragm pumps deliver the consistent flow control that is essential in producing optimal filtrate yields.
Single-use pumps
In today’s evolving manufacturing processes, quaternary diaphragm pumps are also rapidly becoming a first-choice technology in increasingly popular single-use production setups. Basically, a single-use pump enables biopharmaceutical manufacturers to eliminate the cost of cleaning and validating their pumps by using a pump with a replaceable pump head. The result is not only a quicker production process, but one that delivers preferred levels of product purity and sterility with no chance for cross-batch or cross-product contamination (Figure 4).

Figure 4. Among the advantages of using a single-use quaternary diaphragm pump, such as the one shown here, is the ability to use one pump head for one production campaign. At the conclusion of the production campaign, the pump chamber that has come in contact with the fluids is disposed of
The following are some additional advantages that can be realized when quaternary diaphragm single-use pumps are used:
- Quaternary diaphragm pumps (single-use) can be used for one product or in one production campaign
- At the conclusion of the production campaign, the pump chamber that has come in contact with the fluids is disposed of
- Can be used for a set amount of time before the wetted parts are replaced, which eliminates elevated maintenance costs
- If the operator needs to use a stainless-steel pump, the plastic pumping chamber can be replaced with a stainless-steel one
- If there’s a pump failure, the old chamber can be taken out and replaced with a new one in five minutes
- Used when cleaning in place (CIP) or steam sterilization is not practical or possible. This represents a significant simplification and cost reduction to the overall process, as there are no contaminated cleaning chemical and water solutions that need to be treated and disposed of. The costs to properly treat and dispose of the cleaning fluids can alone be the driver to require use of single-use alternatives
Of course, not every pump technology is completely perfect for every characteristic of a specific fluid-handling application. In this instance, the design and operation of the quaternary diaphragm pump limits it to handling fluids that have a maximum viscosity of 1,000 centipoise (cP) and that contains particulates up to 0.1 mm in diameter. n
References
1. Pall Corp., www.pall.com/main/biopharmaceuticals/product.page?id=33058
2. Hagel, L., Jagschies, G., and Sofer, G., “Handbook of Process Chromatography: Development, Manufacturing, Validation and Economics,” Academic Press, Elsevier, 1997.
3. Aranha, H. and Forbes, S., Viral Clearance Strategies for Biopharmaceutical Safety, Pharmaceutical Technology, June 2001.
Glenn Hiroyasu is the Americas Development Manager for Quattroflow Fluid Systems GmbH, a brand of Almatec Maschinenbau GmbH (Carl-Friedrich-Gauß-Straße 5, 47475 Kamp-Lintfort, Germany; Phone: +49-2842-961-0; fax: +49-2842-961-40), which is a product brand of PSG (1815 S. Meyers Road, Oakbrook Terrace, IL, 60181; Phone: +1-630-487-2240; Fax; +1-630-487-2250; Email: [email protected]), a Dover company. Hiroyasu has 28 years of experience in filtration and downstream processing, and has held sales, marketing and applications positions with Pall, Smartflow and Sartorius Corp. He holds a B.S. in biology and a B.S. in management and human resources from California State Polytechnic University, Pomona.
 Sueli Roel Backes is business development manager, EMEA Hygienic segment at PSG and Almetec Maschinenbau GmbH (Carl-Friedrich-Gauß-Straße 5, 47475 Kamp-Lintfort, Germany; Phone: +49-2842-961-0; Fax: +49-2842-961-40; Email: [email protected]). Roel Backes has over 25 years of experience in the hygienic market, starting with filling lines at Krones AG, then process systems at Van der Molen GmbH, and now pumps with PSG. She holds an M.B.A. degree from FOM University of Applied Science (Munich, Germany).
Sueli Roel Backes is business development manager, EMEA Hygienic segment at PSG and Almetec Maschinenbau GmbH (Carl-Friedrich-Gauß-Straße 5, 47475 Kamp-Lintfort, Germany; Phone: +49-2842-961-0; Fax: +49-2842-961-40; Email: [email protected]). Roel Backes has over 25 years of experience in the hygienic market, starting with filling lines at Krones AG, then process systems at Van der Molen GmbH, and now pumps with PSG. She holds an M.B.A. degree from FOM University of Applied Science (Munich, Germany).